Many providers are moving beyond epidural steroid injections (ESIs) for patients with chronic low back pain associated with lumbar spinal stenosis (LSS).
Instead of simply masking the pain caused by an enlarged ligament with epidural injections, which may only provide temporary pain relief, providers now opt for more innovative and durable spinal stenosis treatment options such as the mild® Procedure.

An epidural steroid injection, which is a medication that is injected into the epidural space in the lower spine to reduce swelling and offer pain relief, may be offered to patients with chronic low back pain from conditions such as lumbar spinal stenosis.
Recent data indicates that repeat epidural injections for patients who experience only short-term improvement may not be in the patient’s best interest in the long term. Alternative treatments, such as minimally invasive lumbar decompression, or the mild® Procedure, may be a better option for some patients.

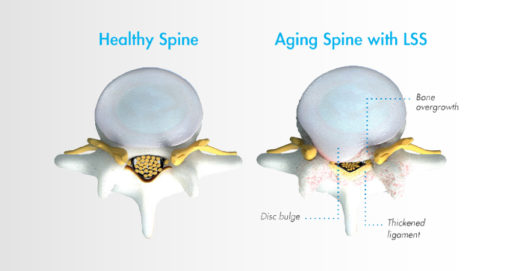
Lumbar spinal stenosis, also called LSS, contributes to chronic low back pain and is prevalent in approximately 20 percent of patients over the age of 60. LSS is often caused by an enlarged ligament in the back, which compresses the space around the spinal canal and puts pressure on the nerves in the lower back. This pressure around the spinal cord can cause pain, numbness, heaviness, or tingling in the low back, legs, and buttocks. A common visual cue is often referred to as the “shopping cart syndrome,” where the act of leaning over, often over a shopping cart, cane, or walker, helps to temporarily alleviate pressure felt in the lower back pain.
In addition to epidural steroid injections, some common conservative treatment options for LSS can include the mild® Procedure, medication, and/or physical therapy, with more invasive options including procedures such as spacer implants, spinal stenosis surgery, or other open surgery.
Epidural steroid injections are typically offered to LSS patients when more conservative treatment options, such as exercise and physical therapy, have failed to provide relief.
Steroid medication is injected directly into the epidural space, which may relieve pain by reducing inflammation around the spinal cord and nerves. The effects typically last for less than 6 months, after which additional injections may need to be administered.
Data shows that epidural steroid injections can effectively relieve pain for LSS patients—but the effects are not lasting, and pain may return, typically in months. ESIs treat the symptoms but do not address the root cause of pain associated with LSS.
While ESIs are an effective form of early treatment for some patients, they may not provide reliable, lasting relief for all low back pain.
As mentioned in the Best Practices for Minimally Invasive Lumbar Spinal Stenosis Treatment 2.0 (MIST), certain payer guidelines, including Centers for Medicare and Medicaid Services (CMS), now stipulate that patients should have obtained a minimum of 3 months of pain relief with eventual recurrence of pain before it is reasonable to proceed with additional injection therapy.
This means that for patients exhibiting shorter-term relief of less than 3 months after receiving an ESI, clinicians should consider alternative treatment options.
Steroid medication reduces inflammation, which can temporarily relieve pain. However, epidural steroid injections only treat the symptoms of LSS—not the root causes of pain and inflammation. The effects of epidural steroid injections typically last less than 6 months, and patients often require an average of 2–3 injections per year to sustain long-term relief from low back pain associated with LSS.

There are many patients for whom repeat epidural steroid injections may offer more risks than benefits. For instance, steroid medications have been linked to bone loss, or osteoporosis. ESIs may also introduce risks for patients with certain comorbidities such as diabetes, cardiovascular conditions, active infections, bleeding disorders, or those taking anticoagulant medications.
As an alternative, epidural injections without the use of steroids may be considered, as well as more advanced decompressive therapies such as the mild® Procedure.
In addition to the health concerns associated with repeat steroid injections, the mental and emotional effects experienced by many LSS patients can also reveal the dark side of repeat epidural steroid injection treatments.
Due to the temporary nature of epidural steroid injection relief and the requirement for repeat injections, many practices encounter patients with what is increasingly becoming known as “ESI Exhaustion.” ESI Exhaustion can be spotted in patients at any stage of LSS treatment or stenosis severity.

When patients experience short-term relief for a condition as challenging as LSS, they can easily become frustrated and lose hope. LSS patients often experience debilitating pain and loss of mobility that can have a devastating impact on their outlook and optimism for the future. Losing additional time and energy to repeated appointments, procedures, and recovery times can also be detrimental to their quality of life, and some patients may start to feel hopeless if injections remain ineffective or lose their efficacy soon after receiving them.
One of the more common questions patients have about a steroid injection is, “How long will the results last?” Unfortunately, with epidural steroid injections, efficacy can vary by patient, and it can be difficult to predict the degree of relief or durability of effect for the individual. While studies have shown symptom relief for up to 6 months in some lumbar spinal stenosis patients, other studies have demonstrated the limited effectiveness of epidural steroid injections.
If patients are dissatisfied with their results and feel they have run out of options in your practice, they may search for another solution. By offering alternative treatments such as the mild® Procedure as an early intervention, you can retain the patients in your practice and increase productivity, while continuing to develop closer relationships and increase your reach within your community.
Given the significant advances in minimally invasive spine technology, current research confirms that repeat epidural steroid injections should be reserved only for patients who experience significant and lasting relief after the injections, and/or those who are not candidates for higher-level interventions or surgical decompression.
For patients experiencing relief that lasts fewer than 3 months, clinicians may wish to consider more durable treatment options.
While they may offer temporary relief, epidural steroid injections do not “cure” LSS. Without addressing the enlarged ligament, which contributes up to 85% of spinal canal narrowing , relief may only be experienced on a short-term or temporary basis.
Minimally invasive lumbar decompression may be the next step for long-lasting relief from LSS and to reduce pressure in the canal. By decreasing the amount of space taken up by the enlarged ligament, patients can experience decreased pressure on the spinal nerves, which may lead to decreased pain.
Performing multiple epidural steroid injections only delays patients from receiving treatment with more lasting results, such as minimally invasive lumbar decompression—the mild® Procedure.
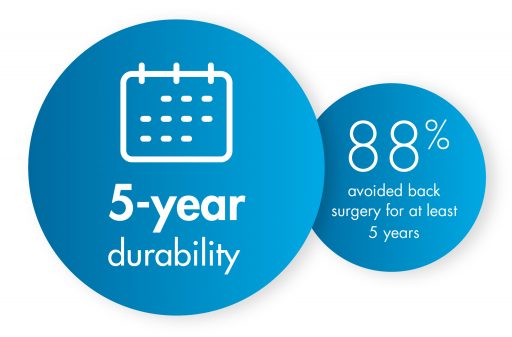
Turning to mild® as the first line of therapy addresses the root cause of LSS by removing excess ligament tissue around the spine, proven to provide a 5-year durability of results.

The mild® Procedure is a short, outpatient procedure that can be performed using only local anesthetic and light sedation. The procedure is performed through a single incision in the low back smaller than the size of a baby aspirin, or the diameter of a drinking straw (5.1mm).
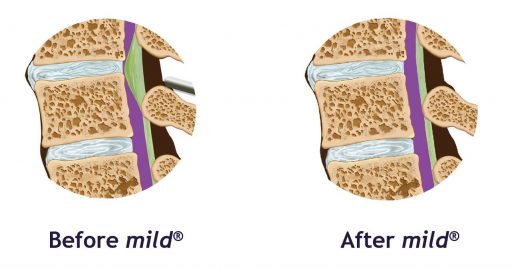
By removing excess ligament tissue that has built up around the spine, mild® restores space in the spinal canal. This reduces pressure on the nerves in the low back, addressing a major root cause of LSS, which can help reduce pain.

When Epidural Steroid Injections (ESIs) Don’t Provide Lasting Relief, mild® can improve patient outcomes across a variety of measures:
In a study performed at the Cleveland Clinic 1 year after the mild® Procedure, patients were able to:
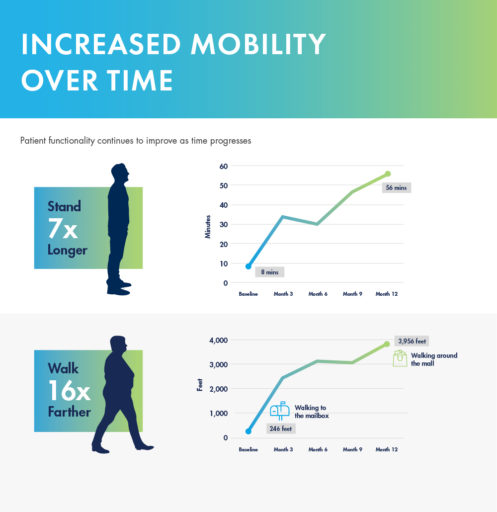
mild® demonstrated excellent long-term durability with significant improvements in both pain and mobility over 2 years. Clinical data from the MiDAS ENCORE 2-Year Study finds mild® provided patients with lasting pain relief and increased mobility.
A 5-year study performed at the Cleveland Clinic demonstrated that mild® helped 88% of patients avoid back surgery for at least 5 years while providing lasting relief. Use our Find a mild® Doctor tool to connect with an interventional pain management specialist in your local area to find out if mild® is right for you.
To learn more about mild® and how it can help people suffering from LSS get on the path to lasting relief, explore mildprocedure.com.
If you experience chronic low back pain (CLBP), you may have questions: What’s causing it? What do my symptoms mean? Will my condition worsen as I age? How can I find relief?
You’re looking for answers—and you’re not alone. Unlike other debilitating conditions, researchers have never truly known how many people suffer from CLBP. Until recently, many patients have been left in the dark about the cause of their pain or their options for treatment.
As revealed in the Mobility Matters: Landmark Survey on Chronic Low Back Pain in America, created in partnership with The Harris Poll, there are many misconceptions about chronic low back pain, including its potential causes, symptoms, and treatment options.
Before this survey, we didn’t know which patients were suffering the most, or how the CLBP experience may change through life’s decades. In this blog, we’ll share the results of the survey, explore a common, yet often undiagnosed, cause of CLBP, and discuss some of the treatment options available for patients seeking relief.
As we grow older, it can be difficult to assess which mobility challenges are a normal part of aging, and which ones may indicate a condition such as CLBP. The Mobility Index, developed as part of the national Know Your Back Story campaign, was designed to demonstrate how older adults could be moving through life if chronic low back or leg pain was not a limiting factor.
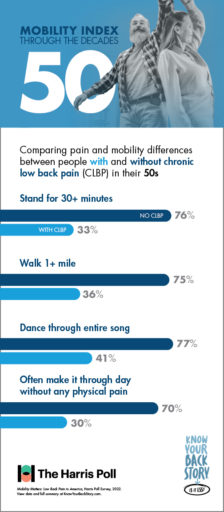
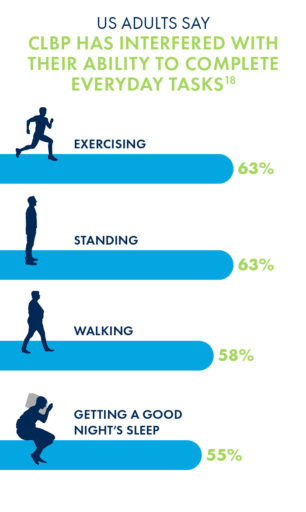
Poll results show that with age, CLBP patients experience significantly greater challenges performing physical tasks and making it through the day without pain than their peers who do not suffer from low back pain.
If you’re suffering from CLBP, you’re already familiar with the limits your pain can put on daily tasks and activities. But do you know just how much you could be doing without these obstacles?

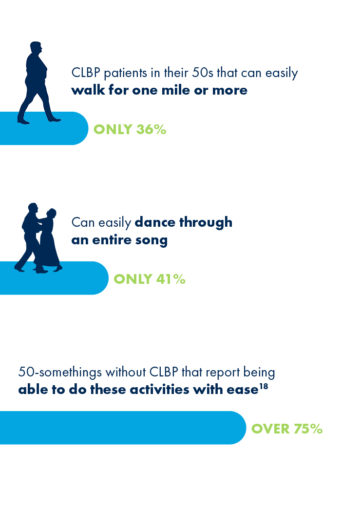
For CLBP patients in their 50s, having difficulty doing physical activities that were once a regular part of life, such as walking a mile or dancing for the duration of one song, can feel especially discouraging.
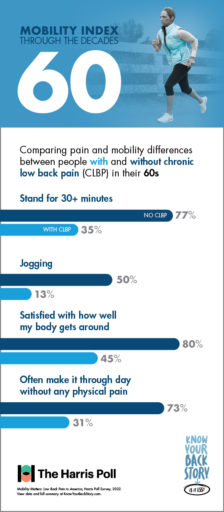
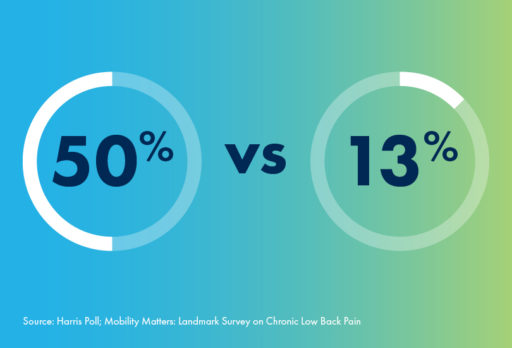
For people in their 60s, there are some activities like—jogging—that aren’t for everyone. Even among individuals without CLBP, only 50% of respondents in their 60s reported the ability to jog with ease. However, for patients suffering with chronic low back pain, this number plummets to only 13%.
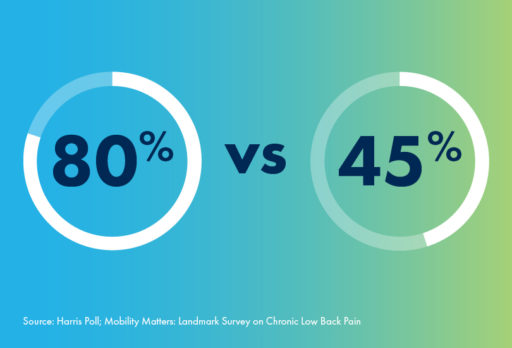
And whether jogging, walking, or doing anything else, fewer than half of CLBP patients in their 60s say they feel satisfied with how their body gets around. In contrast, 80% of 60-somethings without CLBP are satisfied with their mobility.

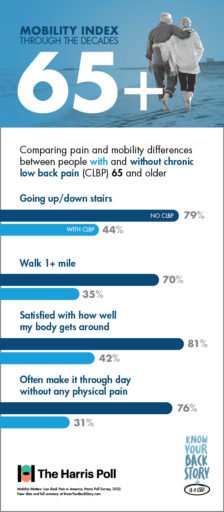
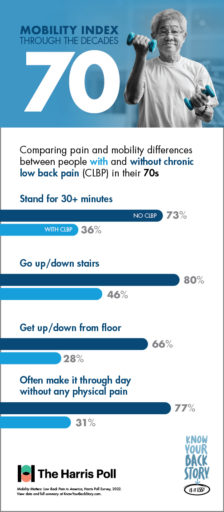
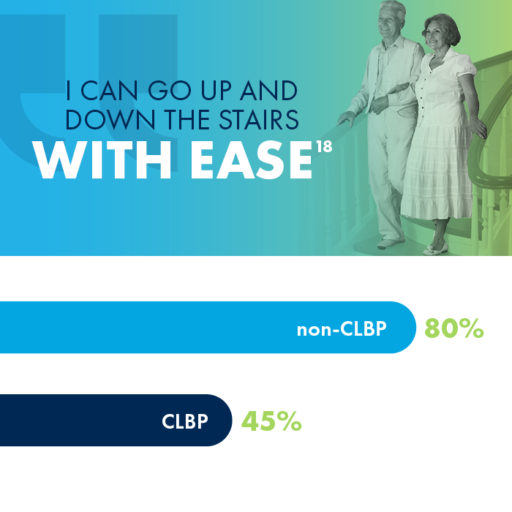
Did you know that 80% of people in their 70s without CLBP are able to easily go up and down the stairs? If you are a CLBP sufferer in your 70s, you may have a much different experience, as fewer than half of CLBP patients in their 70s reported the same mobility using stairs.
Getting up from the floor is another activity that impacts CLBP sufferers much more than their peers who don’t experience chronic pain. While 66% of 70-somethings without CLBP reported ease in getting up or down from the floor, only 28% of those with CLBP were able to say the same.

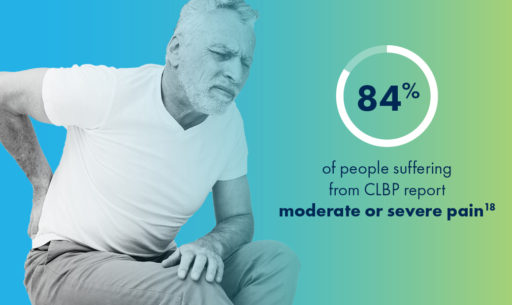
One cause of low back pain that often goes undiagnosed is an enlarged ligament, which can contribute to lumbar spinal stenosis (LSS), a common, yet overlooked, condition that millions of people may be unaware of.

Lumbar spinal stenosis (LSS) is a common, yet overlooked, condition that is prevalent in nearly 20% of patients over the age of 60.
LSS is often caused by an enlarged ligament in the back, which compresses the space in the spinal canal and puts pressure on the nerves in the lower back. This pressure around the spinal cord can cause pain, numbness, heaviness, or tingling in the low back, legs, and buttocks.
Unsurprisingly, the chronic low back pain that may be caused by LSS has negative impacts on nearly every aspect of a patient’s life, most commonly in their abilities to exercise, stand or walk for long periods of time, and get a good night’s sleep.
Due to its minimally invasive nature and long-lasting durability, many interventional pain management doctors are making the move to mild® as an alternative to epidural steroid injections (ESIs), which may only work in the short-term and may require repeat injections to maintain relief.
More invasive courses of treatment can include procedures such as spacer implants or open surgery, though nearly 80% of CLBP sufferers have concerns about undergoing surgery.
The mild® Procedure, or minimally invasive lumbar decompression, is considered a gold standard of care among treatments for low back pain. By addressing the root cause of pain, the enlarged ligament, mild® has helped 88% of patients avoid back surgery for at least 5 years while providing lasting relief.
There’s good news for patients suffering from chronic lower back pain caused by lumbar spinal stenosis (LSS), as effective treatment options have become more widely available. Traditionally, administering a series of epidural steroid injections (ESIs) had been considered the standard of care, but the short-term results—typically lasting less than 6 months—left many doctors and patients looking for a more durable solution. Rather than enduring one injection after another, up to 2-3 injections per year, the minimally invasive mild® Procedure has become an increasingly sought after alternative for patients with LSS.
A recent study published in Future Medicine, highlighted some of the top reasons doctors and patients are making the move to the mild® Procedure, or, minimally invasive lumbar decompression. The mild® Procedure is a treatment option that addresses a major root cause of LSS, requires only local anesthetic and light sedation, and leaves no implant behind.
Here’s an overview about ESIs, the mild® Procedure, and how to find an interventional pain provider capable of telling you more about the procedure and its potential benefits.
Epidural steroid injections—medication injected in the lower spine to reduce swelling and offer pain relief—are typically offered to lumbar spinal stenosis patients when non-medical care methods like exercise and physical therapy have failed to provide relief.
The steroid medication in the injection is believed to reduce inflammation, which relieves pain. However, injections only treat the symptoms of stenosis and do not address the problem’s root cause.
The effects of an epidural steroid injection typically last less than 6 months so patients generally require 2-3 injections per year. Repeat steroid use is known to increase risk of infections and may cause bone loss (osteoporosis).

If epidural steroid injections aren’t effective for you, it’s important to know that your interventional pain provider may offer other treatment options. Proactively discussing and considering different options can help you and your doctor make the right treatment decision at the appropriate time.
The recent study highlighted several advantages of the mild® Procedure, including:

Finding a doctor that offers the mild® Procedure is easy. The fastest way is to use the Find a mild® Doctor feature available on this website. The finder can help you locate a mild® provider in your preferred radius of your address, city, or ZIP code.
When discussing treatment options with your doctor, be sure to ask specific questions, including:
Hear Linda’s story on how mild® relieved her pain and enhanced her everyday life.
The best time to start searching for a mild® Doctor is today. Chronic lower back pain caused by lumbar spinal stenosis typically worsens in severity over time, making early intervention crucial to restoring healthy function, movement, and quality of life. The study discussed here supports early use of the mild® Procedure, finding that:

If you’re ready to learn more about the mild® Procedure, talk to your interventional pain provider or Find a mild® Doctor today.
If you’ve received an epidural steroid injection (ESI) to help manage the pain associated with lumbar spinal stenosis (LSS), but did not experience significant or lasting pain reduction, you are not alone. Although ESIs can be an effective early treatment option for patients with LSS, they are not a one-size-fits-all solution. After an initial epidural injection (or even a series of several injections), many people do not feel significant pain reduction, or many only feel better for a few weeks before the pain returns.
Don’t lose hope—you still have options
Epidurals may not have worked for you, but that does not mean surgery is your only option. Other minimally invasive treatment alternatives, like the mild® Procedure, offer a safe and effective approach that can get you on the path to relief.
When you have spinal stenosis, your lower spinal canal narrows and compresses the spinal nerves in your lower back. This compression can contribute to the pain and mobility issues you are likely experiencing.
In people with LSS, up to 85% of spinal canal narrowing may be caused by a ligament in the back that becomes thickened over time. The mild® Procedure is a minimally invasive treatment option that removes excess ligament tissue to restore space in the spinal canal. The mild® Procedure typically takes less than an hour, and can be performed through a single, tiny incision smaller than the size of a baby aspirin (5.1 mm).
Patients who have been recommended for, or have tried epidural steroid injections may wonder when they should seriously consider an alternative treatment option that can offer effective, lasting pain relief for their LSS. Here are the 3 most common signs that you may be ready to move to mild®, and the steps you can take if you would like to discuss your options with a mild®-trained doctor.
#1: Your first ESI did not significantly reduce your pain
The patients with LSS who are going to benefit from an ESI can typically be identified fairly quickly after the first injection. In fact, a large clinical study completed across several treatment centers found that the level of pain relief patients experienced 6 weeks after their first injection were generally maintained through 12 months, and that repeated injections offered no additional long-term benefit. Stated simply, 6 weeks after your first injection, you can generally tell whether ESIs are likely to work for you.
Jane Hartigan, an Advanced Practice Provider who works as a Physician Assistant (PA) at a leading pain management practice in Northern California, confirms that their practice routinely moves patients to the mild® Procedure if the first epidural fails. “Our patients love mild®, and it’s been a game-changer in our practice,” Jane says. “mild® offers a similar safety profile to ESIs but with long-term results. So we’ll start with an epidural injection, but we’ll schedule a follow-up soon after the injection to determine if it’s working for that patient. In the many cases that patients are not getting pain relief or improvement in their mobility, we will start to seriously consider the mild® Procedure.”
#2: You’re feeling “epidural exhaustion” after a series of ESIs
Many patients who receive a series of epidural injections can develop “Epidural Exhaustion,” especially if the injections provide little relief or decreasing relief over time. Jane says, “Epidural Exhaustion is incredibly common in lumbar spinal stenosis patients. A lot of patients come to us from other practices where they received injection after injection with very little improvement. They’re frustrated and can begin to lose hope that they’ll ever feel better.”
In Jane’s practice, LSS patients can move to mild® within weeks of their first injection if the epidural does not relieve their pain. According to Jane, “Many practices perform multiple ESIs, one after another, but we move more quickly toward mild®. When patients are given multiple injections, I feel like it’s just delaying a much better treatment option for that patient. mild® addresses a major root cause of LSS, it’s proven safe and effective, and our patients have had such amazing results. Seeing patients come in after their mild® Procedure, and they’re just so grateful and excited about finally feeling better and being able to walk or stand. It’s so rewarding for me, and reinforces that we’re taking the right approach.”
#3: You’re looking for long-term relief
A key benefit of the mild® Procedure is that unlike ESIs, mild® offers patients lasting results. Many stenosis patients who do show improvement after an initial epidural injection will notice that the relief is short-lived, and additional injections are needed to sustain relief. This is because ESIs do not address the major root cause of LSS. mild®, on the other hand, removes excess ligament tissue that causes the narrowing of the spinal canal without significantly changing the structural anatomy of the spine.
A recent study conducted by the Cleveland Clinic highlights the long-term relief provided by mild®.
The study found:
“One of the most rewarding aspects of offering mild® in our practice is having patients that come back to us with other issues, but their lower back is still feeling better,” noted Jane. “I’ve had patients come in months and years later, and they feel the same relief they experienced after the mild® Procedure. They have gone on with their lives without worrying about their LSS.”
If you are ready to move on from epidural steroid injections, and to find out if you are a candidate for the mild® Procedure, there are a few steps you can take to put yourself on the path to relief.
Find a mild® doctor in your area

Take charge of your health

Understand the plan

Benyamin RM, Staats PS, MiDAS ENCORE Investigators. mild® is an effective treatment for lumbar spinal stenosis with neurogenic claudication: MiDAS ENCORE Randomized Controlled Trial. Pain Physician. 2016;19(4):229-242.
Mekhail N, Costandi S, Abraham B, Samuel SW. Functional and patient-reported outcomes in symptomatic lumbar spinal stenosis following percutaneous decompression. Pain Pract. 2012;12(6):417-425. doi:10.1111/j.1533-2500.2012.00565.x.
2012 data from Health Market Sciences report for Vertos Medical 2013.
Data on file with Vertos Medical.
Staats PS, Chafin TB, Golvac S, et al. Long-term safety and efficacy of minimally invasive lumbar decompression procedure for the treatment of lumbar spinal stenosis with neurogenic claudication: 2-year results of MiDAS ENCORE. Reg Anesth Pain Med. 2018;43:789-794. doi:10.1097/AAP.0000000000000868.
Based on mild® Procedure data collected in all clinical studies. Major complications are defined as dural tear and blood loss requiring transfusion.
MiDAS ENCORE responder data. On file with Vertos Medical.
Jain S, Deer TR, Sayed D, et al. Minimally invasive lumbar decompression: a review of indications, techniques, efficacy and safety. Pain Manag. 2020;10(5). https://doi.org/10.2217/pmt-2020-0037. Accessed June 1, 2020.
Deer TR, Grider JS, Pope JE, et al. The MIST Guidelines: the Lumbar Spinal Stenosis Consensus Group guidelines for minimally invasive spine treatment. Pain Pract. 2019;19(3)250-274. doi:10.1111/papr.12744.
Hansson T, Suzuki N, Hebelka H, Gaulitz A. The narrowing of the lumbar spinal canal during loaded MRI: the effects of the disc and ligamentum flavum. Eur Spine J. 2009;18(5):679-686. doi:10.1007/s00586-009-0919-7.
Treatment options shown are commonly offered once conservative therapies (e.g., physical therapy, pain medications, chiropractic) are not providing adequate relief. This is not intended to be a complete list of all treatments available. Doctors typically recommend treatments based on their safety profile, typically prioritizing low risk/less aggressive procedures before higher risk/more aggressive procedures, but will determine which treatments are appropriate for their patients.
The mild® Procedure is a minimally invasive treatment for lumbar spinal stenosis. As with most surgical procedures, serious adverse events, some of which can be fatal, can occur, including heart attack, cardiac arrest (heart stops beating), stroke, and embolism (blood or fat that migrates to the lungs or heart). Other risks include infection and bleeding, spinal cord and nerve injury that can, in rare instances, cause paralysis. This procedure is not for everyone. Physicians should discuss potential risks with patients. For complete information regarding indications for use, warnings, precautions, and methods of use, please reference the devices’ Instructions for Use.
Patient stories on this website reflect the results experienced by individuals who have undergone the mild® Procedure. Patients are not compensated for their testimonial. The mild® Procedure is intended to treat lumbar spinal stenosis (LSS) caused by ligamentum flavum hypertrophy. Although patients may experience relief from the procedure, individual results may vary. Individuals may have symptoms persist or evolve or other conditions that require ongoing medication or additional treatments. Please consult with your doctor to determine if this procedure is right for you.
Reimbursement, especially coding, is dynamic and changes every year. Laws and regulations involving reimbursement are also complex and change frequently. Providers are responsible for determining medical necessity and reporting the codes that accurately describe the work that is done and the products and procedures that are furnished to patients. For this reason, Vertos Medical strongly recommends that you consult with your payers, your specialty society, or the AMA CPT regarding coding, coverage and payment.
Vertos Medical cannot guarantee coding, coverage, or payment for products or procedures. View our Billing Guide.
Vertos is an equal employment opportunity workplace committed to pursuing and hiring a diverse workforce. We strive to grow our team with highly skilled people who share our culture and values. All qualified applicants will receive consideration for employment without regard to sex, age, color, race, religion, marital status, national origin, ancestry, sexual orientation, gender identity, physical & mental disability, medical condition, genetic information, veteran status, or any other basis protected by federal, state or local law.
Hall S, Bartleson JD, Onofrio BM, Baker HL Jr, Okazaki H, O’Duffy JD. Lumbar spinal stenosis. Clinical features, diagnostic procedures, and results of surgical treatment in 68 patients. Ann Intern Med. 1985;103(2):271-275. doi:10.7326/0003-4819-103-2-271.
Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence & association with symptoms: The Framingham Study. Spine J. 2009;9(7):545-550. doi:10.1016/j.spinee.2009.03.005.
Fukusaki M, Kobayashi I, Hara T, Sumikawa K. Symptoms of spinal stenosis do not improve after epidural steroid injection. Clin J Pain. 1998;14(2):148-151. doi:10.1097/00002508-199806000-00010.
Mekhail N, Costandi S, Nageeb G, Ekladios C, Saied O. The durability of minimally invasive lumbar decompression procedure in patients with symptomatic lumbar spinal stenosis: Long-term follow-up [published online ahead of print, 2021 May 4]. Pain Pract. 2021;10.1111/papr.13020. doi:10.1111/papr.13020
Friedly JL, Comstock BA, Turner JA, et al. Long-Term Effects of Repeated Injections of Local Anesthetic With or Without Corticosteroid for Lumbar Spinal Stenosis: A Randomized Trial. Arch Phys Med Rehabil. 2017;98(8):1499-1507.e2. doi:10.1016/j.apmr.2017.02.029
Pope J, Deer TR, Falowski SM. A retrospective, single-center, quantitative analysis of adverse events in patients undergoing spinal stenosis with neurogenic claudication using a novel percutaneous direct lumbar decompression strategy. J Pain Res. 2021;14:1909-1913. doi: 10.2147/JPR.S304997
Pryzbylkowski P, Bux A, Chandwani K, et al. Minimally invasive direct decompression for lumbar spinal stenosis: impact of multiple prior epidural steroid injections [published online ahead of print, 2021 Aug 4]. Pain Manag. 2021;10.2217/pmt-2021-0056. doi:10.2217/pmt-2021-0056
Abstract presented at: American Society of Pain and Neuroscience Annual Conference; July 22-25, 2021; Miami Beach, FL.
Mobility Matters: Low Back Pain in America, Harris Poll Survey, 2022. View data and full summary here.
Deer TR, Grider JS, Pope JE, et al. Best Practices for Minimally Invasive Lumbar Spinal Stenosis Treatment 2.0 (MIST): Consensus Guidance from the American Society of Pain and Neuroscience (ASPN). J Pain Res. 2022;15:1325-1354. Published 2022 May 5. doi:10.2147/JPR.S355285.