The MOTION Study, a prospective multicenter randomized controlled study, was designed to evaluate the safety and effectiveness of the mild® Procedure alongside with conventional medical management (CMM) as a first-line therapy in contrast to patients treated with CMM-Alone.
In July 2024, Sherif Costandi, MD, from the Cleveland Clinic shared new MOTION Study data at the American Society of Pain and Neuroscience (ASPN) Annual Conference in Miami, FL. This update compared the 2-year functional outcomes of the CMM-Alone patients who crossed over to mild® with the mild®+CMM as first line treatment group.
Data on subjective and objective measures demonstrate that improvements in patients who received the mild® Procedure after first receiving CMM for up to 1 year are comparable to those in the first-line therapy group (mild®+CMM).
The MOTION Study, a prospective multicenter randomized controlled study, was designed to evaluate the safety and effectiveness of the mild® Procedure alongside with conventional medical management (CMM) as a first-line therapy in contrast to patients treated with CMM-Alone.
In July 2024, Sherif Costandi, MD, from the Cleveland Clinic shared new MOTION Study data at the American Society of Pain and Neuroscience (ASPN) Annual Conference in Miami, FL. This update compared the 2-year functional outcomes of the CMM-Alone patients who crossed over to mild® with the mild®+CMM as first line treatment group.
Data on subjective and objective measures demonstrate that improvements in patients receiving the mild® Procedure after first receiving CMM up to 1 year are comparable to those in the first-line therapy group (mild®+CMM).
Dr. Costandi Explores “Two-Year Follow-Up of Crossover Patients Treated with the mild® Procedure”
(00:00) Hello everyone. This is Sherif Costandi. I’m the Pain Medicine Fellowship Director of the Cleveland Clinic. I’m very excited to share with everyone the results of the two years results of the crossover group of MOTION study. Historically, symptomatic spinal stenosis has been treated first with conservative measures that included oral medications, physical therapy, and possible interventions like epidural steroid injections. Those who failed conservative measures were considered for
(00:30) open surgery decompression. With the inception of mild® for the percutaneous image guided lumbar decompression procedure, this algorithm has been challenged and the mild® positioned itself in the middle for those patients who have failed conservative care measures. However, they’re not really candidates for the open surgical decompression or are not willing to proceed for the surgical decompression surgeries. MOTION study is a level one, RCT, multicentric, five year study.
(01:00) The goal of the study was to evaluate mild® as a first line therapy. The design was to randomly assign patients into two cohorts. A group would be receive conservative medical management alone versus another cohort who would receive mild® as a first line therapy, along with conservative medical measures. We had 72 who were enrolled in the mild®,
(01:30) along with CMM versus 76 patients who were enrolled in the CMM alone. After one year, patients were allowed to crossover from the CMM alone and receive the mild®. What we did in this analysis is we looked at the two years outcomes of those patients who crossed over, and then we compared them to the years outcomes of the patients who had mild® with a CMM as a first line therapy. The outcomes that were measured included numeric pain scores.
(02:00) Patient reported measures like as Oswestry Disability Index, Zurich Claudication questionnaire specific to spinal stenosis and walking tolerance test where patients are asked to walk for 15 minutes at their own pace until they either feel hurt or they start to develop some symptoms, they have to stop or they would stop at the 15 minutes. When we looked at the measures, there was no
(02:30) statistical significance between the CMM alone group and the mild®, the patients that received the mild® as a first line therapy. We look at the median improvement of the ODI in the crossover group as 14.3 versus 16.4 in the mild®, along with the CMM. We look at the Zurich Claudication questionnaires. The symptom severity was 0.8 in the crossover versus 0.8 in the mild®, along with the CMM measures. Same thing with the physical function.
(03:00) It was 0.5 versus 0.5 in the mild® and CMM alone. The walking tolerance test was 209% for the crossover versus 222% for the mild® plus the CMM alone. So there was no statistical significance in any of the means improvements in any of the measures that were assessed. Therefore, the patients that had the conservative measures and then received the mild® procedure had similar outcomes to the patients
(03:30) who received the mild® procedure as a first line therapy. With the safety profile of mild® procedure that’s comparable to epidural steroid injections and the plethora of the studies that showed the effectiveness of the mild®, the algorithm of care for symptomatic spinal stenosis is really challenged and we wonder if the mild® could be considered as a first-line therapy. Thank you everyone, and hope you enjoy a great conference. Thank you.
The views and opinions expressed in this article are those of the authors/speakers and do not necessarily reflect the official policy or position of Vertos Medical.
The MOTION Study is a 5-year prospective, multi-center, randomized controlled study designed to assess the safety and effectiveness of the mild® Procedure alongside with conventional medical management (CMM) as a first-line therapy (treatment group) compared to CMM-Alone (control group).
At the American Society of Pain and Neuroscience (ASPN) Annual Conference in Miami, FL in July 2024, Timothy R. Deer, MD, from The Spine & Nerve Centers of the Virginias shared 3-year follow-up results of the MOTION Study. Data included subjective and objective measures of the group receiving the mild® Procedure (mild® + CMM).
The data provides additional evidence of the durability and safety of mild® as a first-line treatment in a real-world setting, suggesting that while CMM manages symptoms, mild® addresses a primary cause of lumbar spinal stenosis (LSS) by debulking the thickened ligamentum flavum and reducing the compression of neural elements.
(00:00) Hello friends, Tim Deer. The MOTION study is a five-year prospective, randomized controlled trial comparing minimally invasive lumbar decompression where we go in with a small portal and remove pieces of ligament of the ligamentum flavum, that compresses the spinal sac and causes pain with spinal stenosis versus comprehensive medical management, which includes things like physical therapy, injections, medication, ablations, things we normally do in our spine clinic.
(00:30) The 3-year mark we have 40 patients who really are doing quite well. If you look at the data, the outcome measures we’re looking at, things like ODI, things like numeric rating scale, the Zurich claudication scale, and the really, the walking test of how far you can walk, how far you can stand for 15 minutes. All those tests are statistically positive in favor of minimally invasive lumbar decompression. *Statistically positive compared to baseline.
(00:57) In addition, 92% of patients were able to avoid a larger lumbar opened surgery. And I think that’s phenomenal because the other option for these patients have always been open decompression or fusion or both. And I think to see a reduction and large surgery that often are needed, but in this case we found they often aren’t needed and we’re showing that people are doing quite well without bigger interventions.
(01:20) We’ll be presenting this at the ASPN 6th annual meeting. Hope you can join us. If not look for a publication of the three-year data and we’ll be back again, hopefully reporting the four and five-year data to follow. Thank you very much again. Look for the MOTION study. Look for the ligament when you’re treating patients. I’ll see you in Miami.
The views and opinions expressed in this article are those of the authors/speakers and do not necessarily reflect the official policy or position of Vertos Medical. The data is only for the study group receiving the mild® Procedure (mild®+CMM).
Many providers are moving beyond epidural steroid injections (ESIs) for patients with chronic low back pain associated with lumbar spinal stenosis (LSS).
Instead of simply masking the pain caused by an enlarged ligament with epidural injections, which may only provide temporary pain relief, providers now opt for more innovative and durable spinal stenosis treatment options such as the mild® Procedure.

An epidural steroid injection, which is a medication that is injected into the epidural space in the lower spine to reduce swelling and offer pain relief, may be offered to patients with chronic low back pain from conditions such as lumbar spinal stenosis.
Recent data indicates that repeat epidural injections for patients who experience only short-term improvement may not be in the patient’s best interest in the long term. Alternative treatments, such as minimally invasive lumbar decompression, or the mild® Procedure, may be a better option for some patients.

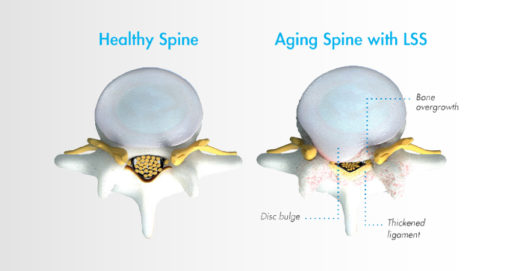
Lumbar spinal stenosis, also called LSS, contributes to chronic low back pain and is prevalent in approximately 20 percent of patients over the age of 60. LSS is often caused by an enlarged ligament in the back, which compresses the space around the spinal canal and puts pressure on the nerves in the lower back. This pressure around the spinal cord can cause pain, numbness, heaviness, or tingling in the low back, legs, and buttocks. A common visual cue is often referred to as the “shopping cart syndrome,” where the act of leaning over, often over a shopping cart, cane, or walker, helps to temporarily alleviate pressure felt in the lower back pain.
In addition to epidural steroid injections, some common conservative treatment options for LSS can include the mild® Procedure, medication, and/or physical therapy, with more invasive options including procedures such as spacer implants, spinal stenosis surgery, or other open surgery.
Epidural steroid injections are typically offered to LSS patients when more conservative treatment options, such as exercise and physical therapy, have failed to provide relief.
Steroid medication is injected directly into the epidural space, which may relieve pain by reducing inflammation around the spinal cord and nerves. The effects typically last for less than 6 months, after which additional injections may need to be administered.
Data shows that epidural steroid injections can effectively relieve pain for LSS patients—but the effects are not lasting, and pain may return, typically in months. ESIs treat the symptoms but do not address the root cause of pain associated with LSS.
While ESIs are an effective form of early treatment for some patients, they may not provide reliable, lasting relief for all low back pain.
As mentioned in the Best Practices for Minimally Invasive Lumbar Spinal Stenosis Treatment 2.0 (MIST), certain payer guidelines, including Centers for Medicare and Medicaid Services (CMS), now stipulate that patients should have obtained a minimum of 3 months of pain relief with eventual recurrence of pain before it is reasonable to proceed with additional injection therapy.
This means that for patients exhibiting shorter-term relief of less than 3 months after receiving an ESI, clinicians should consider alternative treatment options.
Steroid medication reduces inflammation, which can temporarily relieve pain. However, epidural steroid injections only treat the symptoms of LSS—not the root causes of pain and inflammation. The effects of epidural steroid injections typically last less than 6 months, and patients often require an average of 2–3 injections per year to sustain long-term relief from low back pain associated with LSS.

There are many patients for whom repeat epidural steroid injections may offer more risks than benefits. For instance, steroid medications have been linked to bone loss, or osteoporosis. ESIs may also introduce risks for patients with certain comorbidities such as diabetes, cardiovascular conditions, active infections, bleeding disorders, or those taking anticoagulant medications.
As an alternative, epidural injections without the use of steroids may be considered, as well as more advanced decompressive therapies such as the mild® Procedure.
In addition to the health concerns associated with repeat steroid injections, the mental and emotional effects experienced by many LSS patients can also reveal the dark side of repeat epidural steroid injection treatments.
Due to the temporary nature of epidural steroid injection relief and the requirement for repeat injections, many practices encounter patients with what is increasingly becoming known as “ESI Exhaustion.” ESI Exhaustion can be spotted in patients at any stage of LSS treatment or stenosis severity.

When patients experience short-term relief for a condition as challenging as LSS, they can easily become frustrated and lose hope. LSS patients often experience debilitating pain and loss of mobility that can have a devastating impact on their outlook and optimism for the future. Losing additional time and energy to repeated appointments, procedures, and recovery times can also be detrimental to their quality of life, and some patients may start to feel hopeless if injections remain ineffective or lose their efficacy soon after receiving them.
One of the more common questions patients have about a steroid injection is, “How long will the results last?” Unfortunately, with epidural steroid injections, efficacy can vary by patient, and it can be difficult to predict the degree of relief or durability of effect for the individual. While studies have shown symptom relief for up to 6 months in some lumbar spinal stenosis patients, other studies have demonstrated the limited effectiveness of epidural steroid injections.
If patients are dissatisfied with their results and feel they have run out of options in your practice, they may search for another solution. By offering alternative treatments such as the mild® Procedure as an early intervention, you can retain the patients in your practice and increase productivity, while continuing to develop closer relationships and increase your reach within your community.
Given the significant advances in minimally invasive spine technology, current research confirms that repeat epidural steroid injections should be reserved only for patients who experience significant and lasting relief after the injections, and/or those who are not candidates for higher-level interventions or surgical decompression.
For patients experiencing relief that lasts fewer than 3 months, clinicians may wish to consider more durable treatment options.
While they may offer temporary relief, epidural steroid injections do not “cure” LSS. Without addressing the enlarged ligament, which contributes up to 85% of spinal canal narrowing , relief may only be experienced on a short-term or temporary basis.
Minimally invasive lumbar decompression may be the next step for long-lasting relief from LSS and to reduce pressure in the canal. By decreasing the amount of space taken up by the enlarged ligament, patients can experience decreased pressure on the spinal nerves, which may lead to decreased pain.
Performing multiple epidural steroid injections only delays patients from receiving treatment with more lasting results, such as minimally invasive lumbar decompression—the mild® Procedure.
Turning to mild® as the first line of therapy addresses the root cause of LSS by removing excess ligament tissue around the spine, proven to provide a 5-year durability of results.

The mild® Procedure is a short, outpatient procedure that can be performed using only local anesthetic and light sedation. The procedure is performed through a single incision in the low back smaller than the size of a baby aspirin, or the diameter of a drinking straw (5.1mm).
By removing excess ligament tissue that has built up around the spine, mild® restores space in the spinal canal. This reduces pressure on the nerves in the low back, addressing a major root cause of LSS, which can help reduce pain.

When Epidural Steroid Injections (ESIs) Don’t Provide Lasting Relief, mild® can improve patient outcomes across a variety of measures:
In a study performed at the Cleveland Clinic 1 year after the mild® Procedure, patients were able to:
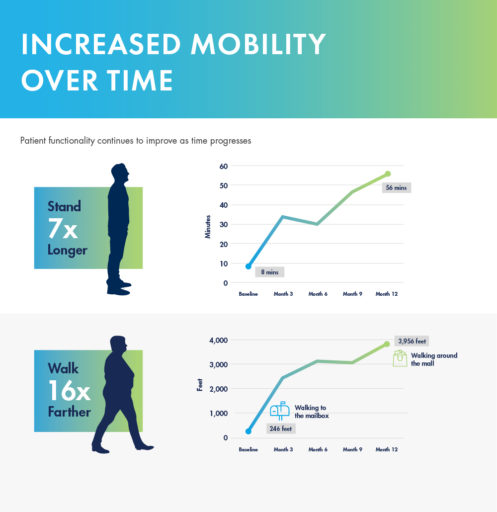
mild® demonstrated excellent long-term durability with significant improvements in both pain and mobility over 2 years. Clinical data from the MiDAS ENCORE 2-Year Study finds mild® provided patients with lasting pain relief and increased mobility.
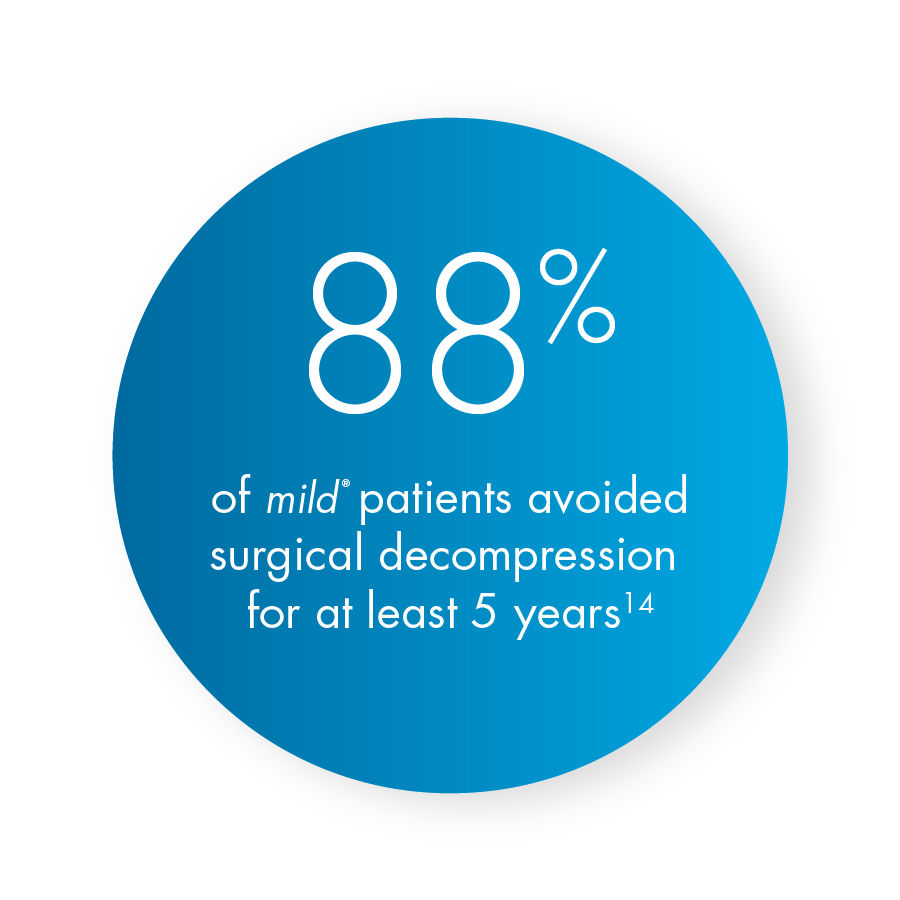
A 5-year study performed at the Cleveland Clinic demonstrated that mild® helped 88% of patients avoid back surgery for at least 5 years while providing lasting relief. Use our Find a mild® Doctor tool to connect with an interventional pain management specialist in your local area to find out if mild® is right for you.
To learn more about mild® and how it can help people suffering from LSS get on the path to lasting relief, explore mildprocedure.com.
In July 2023, the American Society of Pain and Neuroscience (ASPN) held its 5th annual conference in Miami Beach, FL. The society’s goal is to facilitate the role of evidence-based pain medicine research, patient education, and advocacy, and to continue to be on the cutting edge of pain medicine interventions. As part of the conference, a number of abstracts showcased the mild® Procedure, a minimally invasive procedure that debulks thickened ligament in the spinal canal to address a major root cause of lumbar spinal stenosis (LSS). Below are the featured abstracts and their summaries.
Please view the abstracts to review their associated clinical data.
WINNER — Selected as an ASPN Top Abstract 2023
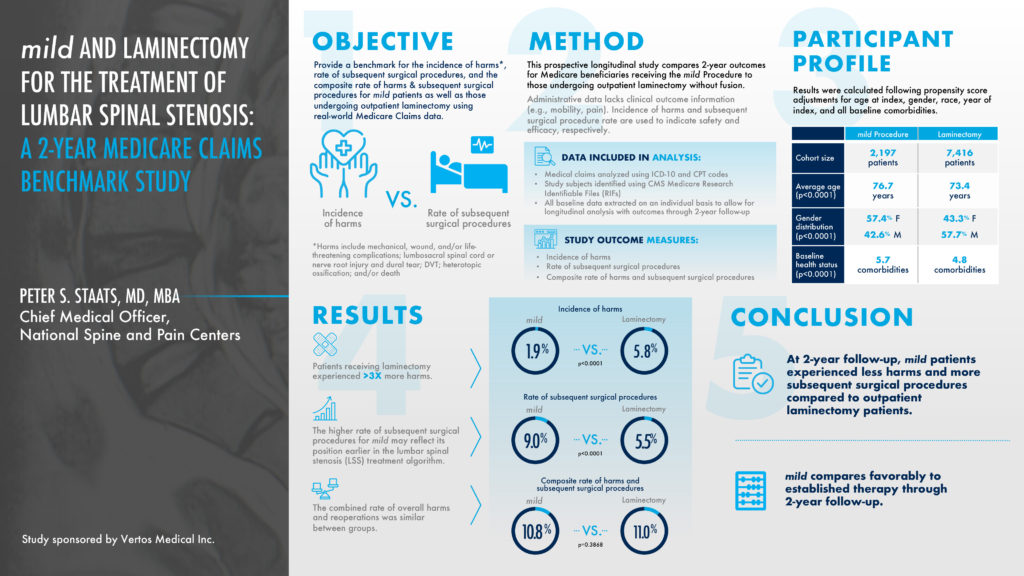
This prospective longitudinal study compared 2-year outcomes for Medicare beneficiaries receiving the mild® Procedure to those undergoing an outpatient laminectomy, a type of spinal surgery. The objective was to provide a benchmark for the incidence of harms versus the rate of subsequent spinal surgical procedures. Patients receiving a laminectomy experienced 3 times more harms at a 5.8% rate, compared to a 1.9% rate for the mild® Procedure. At a 2-year follow-up, mild® patients experienced less harms and more subsequent spinal stenosis surgical procedures compared to outpatient laminectomy patients. The higher rate of subsequent surgical procedures for mild® may reflect its position earlier in the lumbar spinal stenosis treatment algorithm.
This abstract’s objective was to examine and compare the care path, experience, and tradeoff for LSS patients dependent on the treatment they receive, comparing PILD to serial ESIs (epidural steroid injections). As highlighted in the abstract, Percutaneous Image-Guided Lumbar Decompression (PILD) is a proven early treatment option for symptomatic lumbar spinal stenosis resulting from hypertrophic ligamentum flavum (HLF). In contrast, epidural steroid injections (ESIs) typically provide temporary relief in the early stages of LSS but require frequent repetition, delaying improved mobility and comfort. The methodology estimated the 1-year real “costs” for LSS patients including the number of office visits, the number of procedures, the total cost, and patient satisfaction, to name a few.
In terms of office visits, PILD patients typically attend 1—3 visits including a consultation and follow-up visit, whereas serial ESI patients often return for additional injections, increasing their total number of visits to the 5—10+ range. The total cost of multiple visits shows a three-time multiplier for serial ESI patients compared to PILD patients, where the total cost represents both monetary costs like co-pays and transportation, and the time-based opportunity cost such as travel and appointment time. Even the patient satisfaction rate for PILD patients was 11% higher compared to serial ESI patients, 85%—74%.
Due to these differences, PILD offers more patient-centric benefits and fewer costs, and would be recommended:

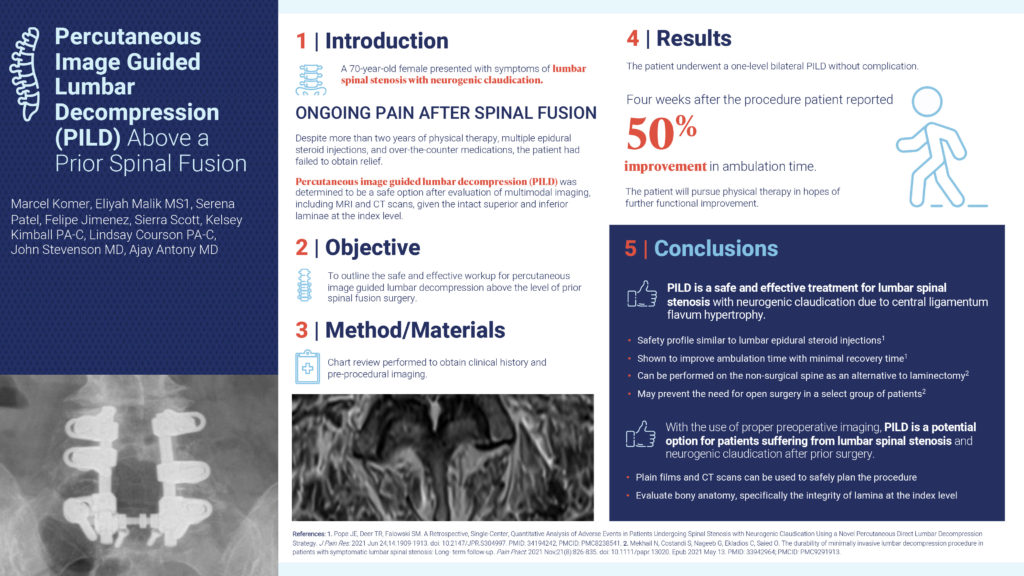
This abstract’s objective was to outline the safe and effective workup for percutaneous image guided lumbar decompression above the level of a prior spinal fusion surgery. In this case study, a 70-year-old female presented with symptoms of lumbar spinal stenosis with neurogenic claudication and felt ongoing pain after a spinal fusion. Despite more than 2 years of physical therapy, multiple epidural steroid injections, and over-the-counter medications, the patient had failed to obtain relief. After an evaluation of MRI and CT scans, the patient underwent a one-level bilateral PILD without complication. Four weeks after the procedure, the patient reported a 50% improvement in ambulation time. In conclusion, with the use of proper preoperative imaging, PILD is a potential option for patients suffering from lumbar spinal stenosis and neurogenic claudication after prior surgery.
This abstract’s objective was to create an approach by which pain practices can increase surgical referrals for PILD by collaborating with surgeon practices, and ultimately optimizing patient care. PILD is an ideal procedure to foster surgeon collaboration since it provides a treatment option for nonsurgical candidates and keeps patient in the practice. Surgical Advanced Practice Providers (APPs) are therefore an ideal PILD referral source as they are familiar with patient histories, have the exposure to nonsurgical candidates, and are eager to optimize patient care. If APPs have knowledge of PILD as a potential treatment, suggesting it as a first option for these nonsurgical patients and collaborating with Interventional Pain Management (IPM) would become routine and enhance patient care.

Authors’ Affiliation
*Novant Spine Specialists, Charlotte, NC, USA
**California University of Science and Medicine, CA, USA
This abstract’s objective was to determine the efficacy and durability of PILD in patients with lumbar spinal stenosis while also implementing a standardized physical therapy referral protocol. These patients were older than 65 years old, with symptoms of neurogenic claudication. They had MRI evidence of at least mild central spinal stenosis with associated ligamentum flavum hypertrophy measuring at least 2.5mm, with a self-reported waning efficacy of epidural steroid injection results. Data points such as standing time and walking distance were collected at a baseline timeframe and 4 weeks post-operatively. At the 4-week follow-up appointment, patients were also referred to a physical therapy routine for core strengthening and endurance building. At the 3-month follow-up, the mean walking distance showed a 986% improvement and the mean standing time showed a 413% improvement. Abstract authors conclude that PILD is a safe and effective treatment option for patients with lumbar spinal stenosis, providing a minimally invasive alternative to traditional spine surgery.

The views and opinions expressed in this article are those of the authors/speakers and do not necessarily reflect the official policy or position of Vertos Medical.
For individuals of any age, mobility is a central wellness indicator that can dramatically impact quality of life. Limited mobility may impact not only physical health and fitness, but also a wide variety of quality-of-life measures, including mental and emotional well-being, social engagement, and access to cultural and recreational experiences.
Despite the importance of mobility, large-scale research that demonstrated how limited mobility due to chronic low back pain (CLBP) impacts patients — relative to their non-CLBP peers — did not exist until recently.
To help more patients suffering from CLBP improve their mobility and quality of life, Vertos Medical, in partnership with leaders in interventional pain management, joined forces with The Harris Poll to understand and educate the public about its impact.
Through their groundbreaking work, an entirely new quality-of-life metric, known as the Mobility Index, was developed. This index can help patients suffering from CLBP understand how their mobility compares to others in their age group who do not experience CLBP.
By combining self-reported data on physical abilities, mobility-related statements, and the completion of everyday tasks, the poll results and Mobility Index offer never-before-available perspectives on how extensively CLBP impacts individuals’ quality of life.
On May 11, 2023, Edward “Paul” Johnson, Vice President of Advanced Analytics from The Harris Poll, and Peter Pryzbylkowski, MD, interventional pain specialist, presented an abstract about the poll data and Mobility Index, “Getting America Mobile: Ways to Improve American Quality of Life,” at the 78th annual American Association for Public Opinion Research (AAPOR) conference.
During the session, the presenters outlined 3 primary goals that guided the development of the survey:
To gauge the impact of a range of health conditions on mobility, including chronic low back pain, a survey was conducted across a diverse sample of over 5,000 adults from non-probability, opt-in panels in the United States.
The collected data was carefully weighted by various demographic factors such as age, gender, race/ethnicity, region, education, household income, household size, and marital status to ensure its representativeness according to the 2021 Current Population Survey.
Among the several health conditions investigated, CLBP emerged as the most prominent cause of decreased mobility, even surpassing prevalent conditions such as anxiety, arthritis, and obesity.

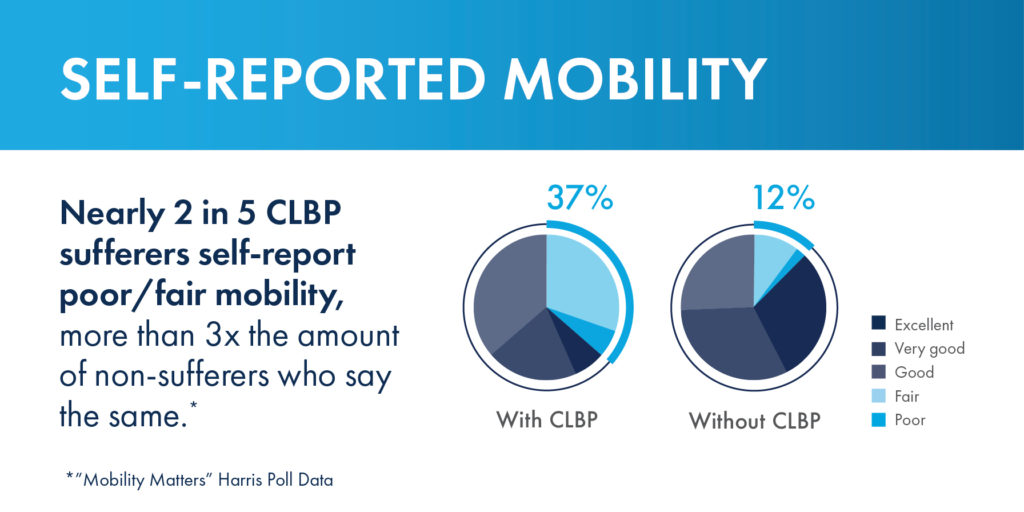
In addition to its utility in measuring mobility and quality of life, the Mobility Index was designed to show patients living with CLBP how they could be moving through the decades of life differently if chronic low back or leg pain was not a limiting factor.
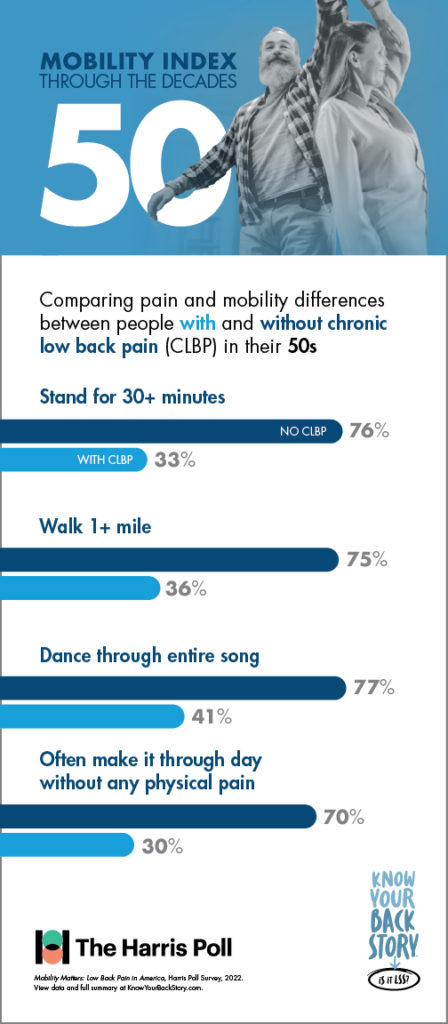
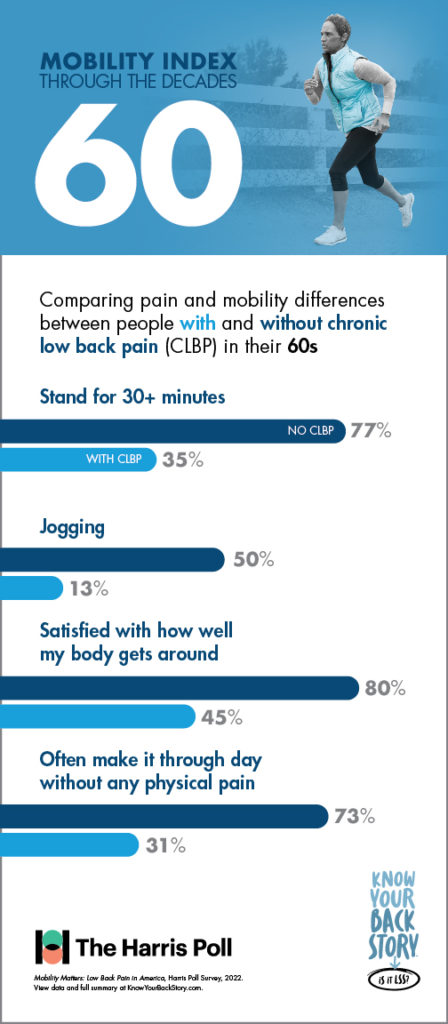
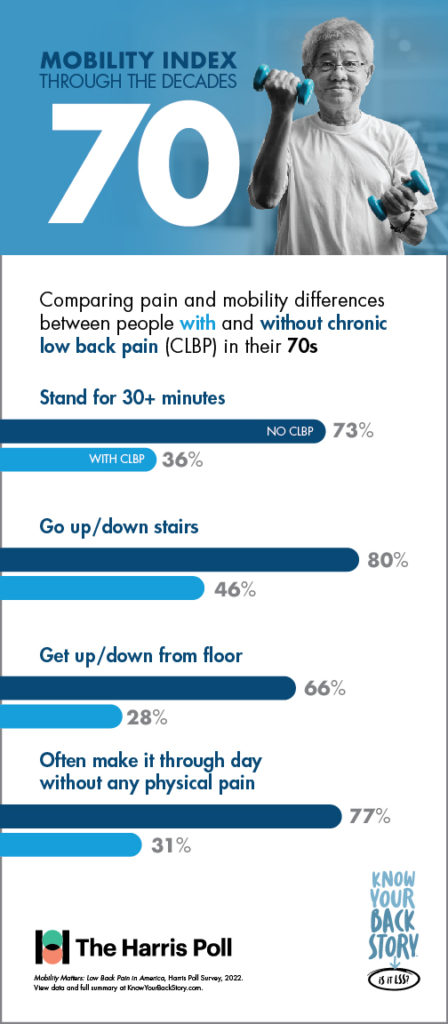

Another concerning disparity revealed in the study was between the prevalence of chronic low back pain and the effectiveness of the most commonly utilized treatment or pain management options.
According to the survey results, up to 15 percent of individuals with CLBP were still relying on opioids , despite updated guidelines from the U.S. Centers for Disease Control (CDC) emphasizing their limited effectiveness and inherent risks.*
Another significant finding was that a mere 5 percent of individuals with CLBP had utilized minimally invasive lumbar decompression treatment options such as the mild® Procedure, despite research indicating that these procedures are highly effective and should be considered earlier in the treatment process.
See More Findings From the Survey
The findings of the Mobility Matters survey were instrumental not only in the development of the Mobility Index but in starting a national public health conversation that spreads awareness about the impacts of CLBP and quality of life.
According to the survey, physicians appear to discuss osteoarthritis or aging as potential causes of CLBP more often than lumbar spinal stenosis, particularly the enlarged ligament that can cause LSS.
By raising awareness about LSS among providers and providing education on the treatment options available for patients suffering from the condition, more patients could get on the path to lasting relief sooner.
“What we found [through the survey] was that CLBP was really a limiting factor when it came to the ability for patients to walk and stand for a prolonged period of time,” Dr. Pryzbylkowski said.
“And that’s important because there are board-certified physicians like myself who treat chronic low back pain on a daily basis. And we just want to bring education and awareness to the general public that there are treatment options for CLBP.”
Through the insights gathered in the Mobility Matters survey, patients, healthcare providers, and policymakers can all gain greater knowledge about the effectiveness of alternative treatment options, the risks associated with opioid use, and the importance of early intervention for CLBP.
Learn more about the Mobility Index and Mobility Matters survey at:
Read the Abstract
References
*Centers for Disease Control and Prevention. Acute low back pain. https://www.cdc.gov/acute-pain/low-back-pain/index.html. Accessed July 21, 2022.
Injections falling short? Advanced IPM practices are moving beyond epidural steroid injections (ESIs) to offer the gold standard of care for lumbar spinal stenosis (LSS) patients. Review the new data where study researchers compared the medical records of participants who had received either just one or no steroid injection prior to the mild® Procedure, to participants who received two or more epidural steroid injections prior to mild®. Similar outcomes in both treatment groups in this study proved that giving more than one ESI prior to the mild® Procedure did not improve how well patients did and may have delayed patient care. Based on the results of the study, it is recommended that the standard treatment process for LSS patients be changed to give the mild® Procedure either as soon as LSS is diagnosed or after the failure of the first ESI.
Congratulations to authors and mild® physicians Peter Pryzbylkowski, Anjum Bux, Kailash Chandwani, Vishal Khemlani, Shawn Puri, Jason Rosenberg and Harry Sukumaran for the first ever plain language article to be published in Pain Management!
View the original article and share the plain language version with your community.




The following study, “mild® Treatment Outcomes in the Presence of Foraminal Narrowing,” evaluates how the mild® Procedure outcomes for patients with hypertrophic ligamentum flavum (HLF) are impacted by the presence of concomitant foraminal narrowing. 413 patients from 11 sites were retrospectively reviewed, with VAS scores compared at baseline and follow-up (1-6 months) within each group over time and between groups. Responder rates were 76.6% for patients with foraminal narrowing, and 78.4% for patients without. Though the amount of pain improvement between groups was not significantly different, this study confirms that by debulking the HLF and reducing compression on the nerves, patients with foraminal narrowing can benefit from a relief in pressure and should not be excluded from patient selection. View the abstract poster below to learn more about the results.

Watch Dr. Navdeep Jassal present his abstract from the New York & New Jersey Pain Medicine Symposium 2021 Conference, where he reviews the data and shares why mild® should be considered for patients impacted by the presence of foraminal narrowing as an adjunctive spinal comorbidity.
Are you ready to put your lumbar spinal stenosis patients on the path to long-term relief? Contact Vertos Medical and discover why leading interventionalists offer mild® in their practice.
Navdeep Jassal, MD (00:00)
My name is Navdeep Jassal. I practice in Lakeland, Florida as an Interventional Pain Physician, and my primary specialty is Physiatry. I’m going to talk a little bit about a poster presentation that will be presented at the New York and New Jersey meeting, talking specifically about the mild® treatment outcomes in patients with presence of foraminal narrowing.
So the onus for this poster was to really evaluate how the mild® Procedure, how their outcomes are, for patients with not only hypertrophic ligamentum flavum, but also how, when you do this mild® Procedure, patients are impacted even with the presence of foraminal narrowing. And we obviously know that this procedure is indicated to those with patients with LSS, with neurogenic claudication, we’re going to look for the ligament. We’re going to look for at least 2.5 millimeters of hypertrophic ligamentum flavum to debulk, but we want to make sure that we understand that physicians aren’t really excluding patients with also other co-factors such as foraminal narrowing. Because oftentimes we realize when you exclude such a patient population, you’re really excluding a patient to receive an outstanding therapy that’s not only safe, but efficacious. And here we actually show a poster to show those types of results.
So we have mild® patients’ recordings that were done across 11 sites or 11 centers with outstanding colleagues. We studied 413 patients retrospectively and out of these 413 patients, we saw 302 patients with foraminal narrowing, and we saw 111 patients with no foraminal narrowing. So if you think about the data, this really shows a typical type of patient population that presents with LSS, those with not just central canal stenosis, but also patients with co-factors that presents with neuroforaminal stenosis, those with facet hypertrophy, but really we’re going to really show that patients actually present, in fact, 302, presents with foraminal narrowing. And we study these patients out, you know, 1-6 months and we followed them up from a VAS (or visual analog scale) perspective. And these were recorded and compared to their baseline findings.
(02:18) From our baseline findings, we saw that these patients typically hovered in the 8 out of 10 or a VAS score of 8 out of 10. And we saw a significant improvement, at least a drop of about 50%. So from an 8 to a 4, about 1-6 months out. What was very interesting about this data specifically, was that there was really no significant difference in terms of the responder rate between those patients that received the treatment of the mild® with those that had foraminal narrowing versus those that did not have foraminal narrowing. In fact, those that had foraminal narrowing in our study were about 76.6% that actually had the responder rate. And then 78.4% that did not have foraminal narrowing had a good responder rate as well. So you can see, there was a very similar responder rate from those that had versus those that did not have foraminal narrowing.
And so from a conclusion standpoint, I really want to make this clear that because both responder rates were similar with no significant difference between patients with or without foraminal narrowing that underwent the mild® Procedure with a significant VAS pain reduction. I really want to hit home that patients with foraminal narrowing can really benefit, from the debulkment of the ligament. And, I think the hypothesis is that when we do this decompression or posterior decompression with the mild® Procedure, we are in fact relieving pressure on the nerves in the central canal. And so again, if we go back to a simple phenomena of a small change in volume is a large change in pressure, we can see how these patients do really well. So again, really trying to hit home that those patients with foraminal narrowing should not be excluded from such a safe and efficacious procedure for their LSS with neurogenic claudication symptoms.
So I think this data is tremendous because it should help those physicians that are skeptical about treating the mild® Procedure for those patients with co-factors. I know I talk to a lot of colleagues who are questioning whether they should offer a mild® Procedure with patients with not only the LFH (ligamentum flavum hypertrophy), but with co-factors of facet hypertrophy, or foraminal stenosis. And I would really challenge them to look at the data and show that, yes you should. If patients have foraminal stenosis or foraminal narrowing, you should treat the patient with the mild® Procedure if they are following signs and symptoms of LSS with neurogenic claudication, and they fit the criteria of something that you can debulk posteriorly with the mild® Procedure. I think it’s an incredibly safe procedure. We’ve proven time and time again, that it’s efficacious from RCTs. And I think this data will challenge you to think more about utilizing the mild® therapy for these patients with LSS.
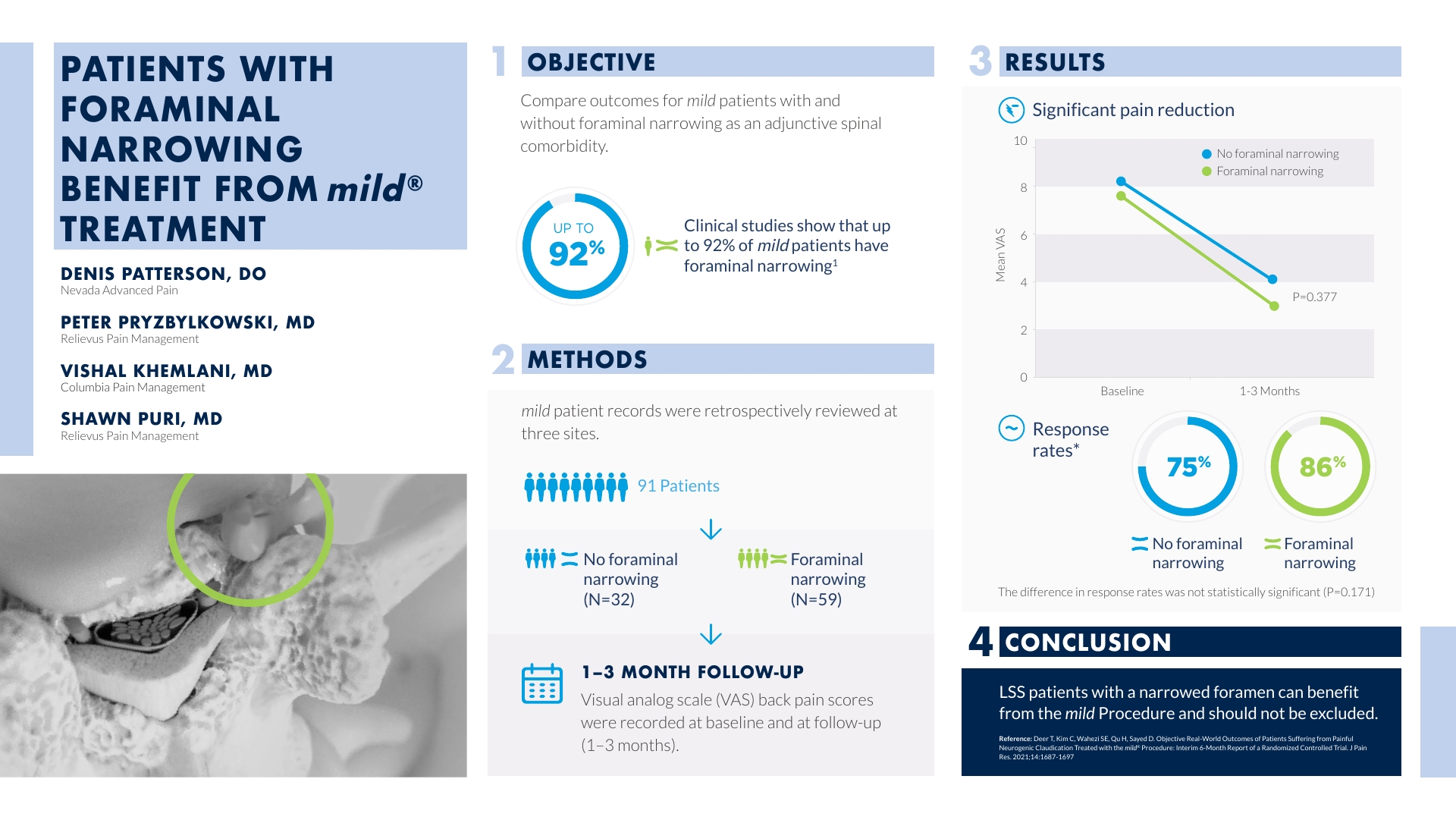
The following study, “Patients with Foraminal Narrowing Benefit from mild® Treatment” investigates procedural outcomes for mild® patients with and without foraminal narrowing as an adjunctive spinal comorbidity. Ninety-one patients from three sites were accepted for analysis and VAS scores were compared within each group over time and between groups. Responder rates were 86.4% in the group with foraminal narrowing, compared to 75.0% in the group having no foraminal narrowing. Though the amount of pain improvement between groups was not significantly different, this study confirms that lumbar spinal stenosis (LSS) patients with a narrowed foramen can benefit from the mild® Procedure and should not be excluded from treatment. View the abstract poster below to learn more about the results.
Watch Dr. Denis Patterson present his abstract from the Pacific Spine & Pain Society (PSPS) 2021 Annual Conference where he reviews the data and shares why LSS patients with a narrowed foramen can benefit from mild®.
Are you ready to put your lumbar spinal stenosis patients on the path to long-term relief? Contact Vertos Medical and discover why leading interventionalists offer mild® in their practice.
Denis G. Patterson, MD (00:00)
Hey, this is Dr. Denis Patterson. I’m a physical medicine rehabilitation physician who specializes in interventional pain management. I am located in the greater Reno, Tahoe area. I’m the owner of Nevada Advanced Pain Specialists. I’ve been seeing and treating patients in the greater Reno, Tahoe area now for about 15 years, and I have to say that one of the best treatment options that I’ve had come around within the past decade has been the mild® Procedure.
Prior to this procedure, as an interventional pain physician, we’re more trying to cover up pain or, alleviate pain, but we never really could treat the underlying pathophysiology. And so it was nice that we finally got a tool in our bag of tricks to help patients that we could actually treat their underlying pathophysiology. So, a patient with wear and tear over the years, not only are they going to get a swollen disc, swollen facet joints, but the ligamentum flavum can also encroach and push into the spinal canal. And that’s what the mild® Procedure does, it allows us to have access to get between the lamina, dig out this ligamentum flavum, create a couple more millimeters of space, and in essence, probably decrease the pressure at that level in their spine and help alleviate their neurogenic claudication symptoms.
Being an advocate for this procedure, I talked to multiple physicians around the country, and one of the interesting comments that I’ve heard over the years is that, “Hey, I can give this or I can treat patients with this, but they can only have central canal stenosis.” And so to me, these physicians are looking for what I would call is a unicorn. A unicorn is a patient who has central canal stenosis only due to the ligament being hypertrophied and that’s simply not the case. When you really look back at the literature and the 2-year study that they did, the MiDAS study, what we see is only 5% of the patients in that study only had central canal stenosis. 95% of those patients had multifactorial stenosis and so that means they can have neuroforaminal stenosis, lateral recess stenosis, and central canal stenosis. And what we saw in the MiDAS study is all those patients benefited.
(02:17) But even though we have that data, over the years of training physicians in the mild® Procedure, I continue to hear, “Well they have neuroforaminal stenosis, they don’t have just central canal stenosis so, I think I’m going to pick a competitive product to treat these patients instead of the mild® Procedure.” So, from the MiDAS data that I had talked about earlier, we’d seen that patients with multifactorial stenosis benefited from the mild® Procedure. Myself, Dr. Pryzbylkowski, and Dr. Khemlani wanted to retrospectively look at patients who we had treated with the mild® Procedure and see if we could validate the results of [what] the MiDAS study had shown. And so we retrospectively reviewed 91 patients from our 3 clinics, and this went back to January of 2020. And what we found is that 32 of the patients that we had treated out of the 91 had no neuroforaminal narrowing. Meaning, they had central canal stenosis, which included ligamentum flavum hypertrophy, but they also could have had multifactorial pathology, including disc bulges and facet hypertrophy. And then the other 59 patients out of the 91, besides having multifactorial pathology, also had neuroforaminal narrowing. And then what we ended up doing is retrospectively looking at our results of these patients, who benefited from the mild® Procedure in these groups; did both groups, 1 group, or neither group benefit? And we looked at our results at one and three months after having the procedure done. And if you look under our results, we look at [how] both groups got significant pain reduction and response rates, what we see is that the foraminal narrowing group did better.
86% of these patients had a positive response rate, while only 75% of the patients that had no neuroforaminal narrowing had a pain reduction. So, when you first look at this, you think, “Oh God, this has got to be statistically significant that the foraminal narrowing group benefited more than the no foraminal narrowing group from the mild® Procedure.” But, when you really look at the “P” value, there is no statistical significance between the 2 groups. And so the conclusion that we came to, is that patients with lumbar stenosis, with neurogenic claudication, whether they have central canal stenosis only, or they have multifactorial stenosis only, both groups can benefit from having the mild® Procedure done. And so, all physicians should know that they should not exclude patients with multifactorial stenosis from having this procedure be considered.
At the American Society of Pain and Neuroscience (ASPN) 2021 meeting in Miami, several abstracts highlighted recent data that supports use of the mild® Procedure as a first line therapy. Additionally, a panel of prominent Interventional Pain Physicians convened to discuss their experiences and clinical pearls for implementing the mild® Procedure in their practices. The consensus? The abstract authors and panelists repeatedly confirmed the rationale, supporting evidence, and benefits of moving to mild® as a first line therapy for lumbar spinal stenosis (LSS).
According to information presented at ASPN, mild® is continuing to be validated as an ideal procedure for LSS patients with neurogenic claudication for several key reasons:
4 speakers on the panel, Drs. Navdeep Jassal, Mark Coleman, Lindsay Shroyer, and David Dickerson, each spoke to the broad patient base that is appropriate for the mild® Procedure.
“For percutaneous image-guided lumbar decompression, any patient with 2.5 millimeters of ligamentum flavum hypertrophy, whether they have central stenosis or lateral stenosis, is a candidate and may achieve pain relief and functional improvement…that’s where I start. The indications are very clear.”
“The fact that they have multilevel disease would lead you towards doing a mild®. A lot of folks were afraid of mild® because of excess radiation, but we can now do these procedures in a fraction of the time that it took in the past. Being able to treat multiple levels of stenosis opens mild® as an option for many more patients.”
“For these procedures, positioning is everything. If you start the procedure with the patient well-positioned, you should be able to access the level you are targeting. Even if you are taking some of the ligament on the opposite side, that can produce good outcomes for the patient. So, for scoliotic curve, there’s not really a maximum or a minimum.”
“This is an elderly patient population. A lot of patients have comorbidities or may be on blood thinners that make them poor candidates for surgery. Why not start with mild®? The excellent safety profile of mild® makes it an excellent option and a procedure I offer to so many of my patients that have lumbar spinal stenosis.”
Clinical approaches that support delivery of the mild® Procedure faster, with less radiation, were a significant focus of the discussions at ASPN. Several leading physicians participated in a clinical study presented by Dr. Dawood Sayed that evaluated the safety and efficacy of the Streamlined Technique compared to the Standard Technique for the Percutaneous Image-Guided Lumbar Decompression (PILD) Procedure.

Their results demonstrated:
In a separate study, Dr. Navdeep Jassal and APP Christine Christensen, MSN, APRN similarly concluded that the Streamlined Technique is a more minimally invasive procedural approach for mild® and is comparable in safety to the Standard Approach, with no increased risk of serious postoperative complications to the patient. Based on this finding, they suggested mild® should be considered the first-line intervention for patients with lumbar spinal stenosis (at least 2.5 mm of hypertrophic ligamentum flavum) and neurogenic claudication after the first epidural steroid injection (ESI) fails.
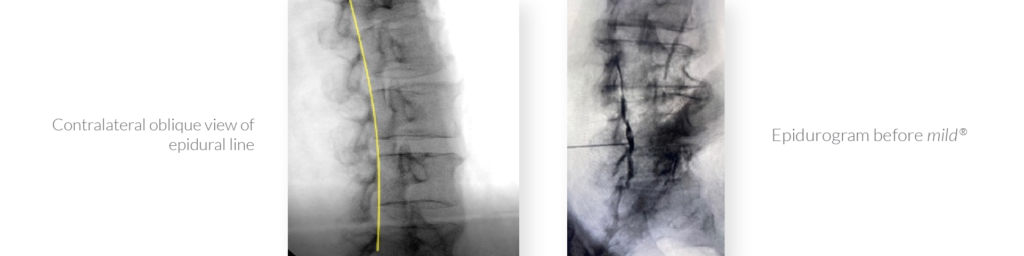
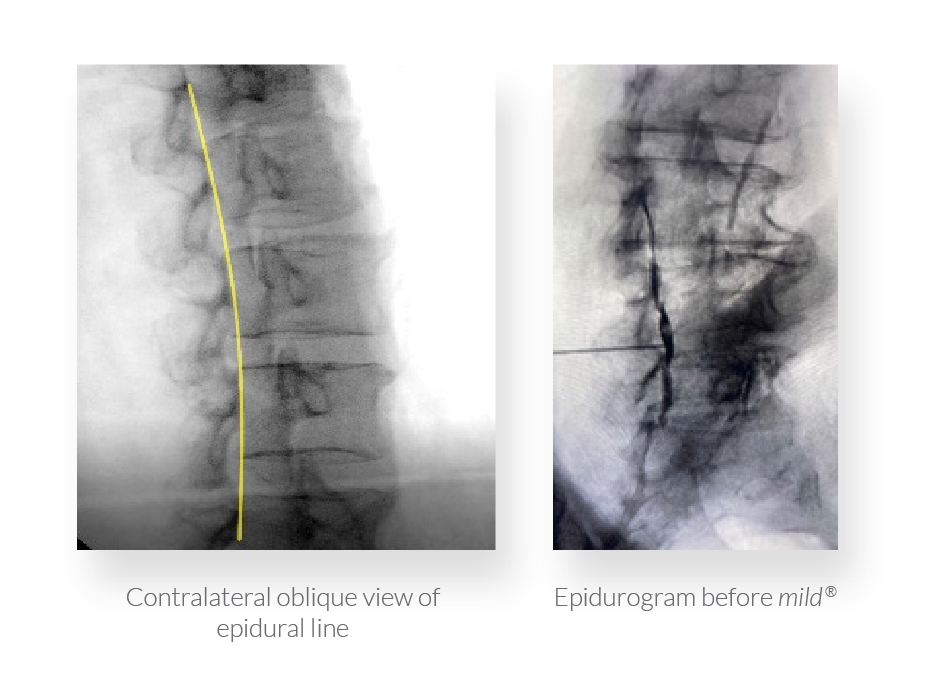
Finally, Drs. Jason Pope, Timothy Deer, and Steven Falowski submitted a poster investigating the safety of using osteal landmarks instead of an epidurogram to establish a visual safety barrier prior to decompression with mild®. Based on zero complications reported across all 147 patients participating in the study, they assert that an epidurogram is not necessary for safe decompression with the mild® Procedure. Contralateral oblique view of the epidural line provides a clear view of the lamina and osteal landmarks, enabling identification of the targeted location for decompression.
Across the panel, numerous speakers highlighted the particular benefits of mild® to patients and urged early integration of mild® in the treatment algorithm.
“mild® has completely changed the way I practice. I implement it early in my algorithm and my patients are really happy. They don’t want surgery, or may have comorbidities that mean they are not a candidate, and they are so excited about their results with mild®. They’ll go out and tell their friends and neighbors, and then we have more patients coming in asking for mild®.”
“When you start doing mild®, the word will spread like wildfire. I’ve done multiple family members who saw the results their loved ones achieved. I tell people, ‘why keep doing epidural steroid injection after epidural steroid injection?’ We know mild® has excellent safety. With mild®, we can give patients good, durable benefits that let them stand, walk, and enjoy a better quality of life.”
“For pain physicians in practices that include a lot of surgeons, start by picking patients who are not good surgical candidates. There are so many patients that may not be ideal for surgery or don’t want surgery that you can help. When you start there, the surgeons in your practice will see the outcomes you’re able to achieve and you can expand from there. This helps smooth the practice dynamics.”
For clinicians considering integrating mild® in their practice armamentarium, or practices already performing the mild® Procedure for failed serial ESI patients or patients who are not candidates for surgery, the data is clear: performing mild® early in the treatment algorithm can offer your LSS patients lasting functional improvements with a safety profile equivalent to ESIs.,
Today, an increasing number of practices recommend their patients move to mild® after the first epidural steroid injection (ESI) fails, and it’s easy to see why. mild® provides patients relief from the symptoms of lumbar spinal stenosis (LSS) and has a safety profile equivalent to an ESI, but with lasting results. In the following article, we present the most common reasons physicians and Advanced Practice Providers (APPs) are moving their patients to mild® earlier in the treatment journey.
Ready to move to mild®? Skip to the end for 3 pragmatic solutions you can implement in your practice today to get started, and get your patients on the path to relief.
A recently published study conducted by the Cleveland Clinic Study explores how many patients were able to avoid surgical decompression after the mild® Procedure over a five-year period.
The study authors concluded that “the durability of mild® over 5 years may allow elderly patients with symptomatic lumbar spinal stenosis to avoid lumbar decompression surgery while providing significant symptomatic relief.” They also noted that “because the mild® Procedure demonstrated durability up to 5 years, it might also be speculated with caution, that appropriate patients should be encouraged to undergo the mild® Procedure as early as needed, rather than waiting until these patients are at an advanced age.”
No implants. mild® removes a major root cause of neurogenic claudication by debulking the hypertrophic ligamentum flavum, which reduces the compression of the nerves without leaving any implants behind.
No stitches. The entire procedure can be performed through a single, tiny incision the size of a baby aspirin (5.1mm).

mild® does not significantly alter the structural anatomy of the spine or eliminate future treatment options
mild® offers a clinically proven safety profile equivalent to an ESI, even in patients with comorbidities.,
Unlike more invasive procedures that can require a lengthy recovery, mild® patients typically resume normal activity within 24 hours with no restrictions.

“When I went for the procedure, it was any other day. I went into the procedure. You feel nothing, and then you come back and you’re fine. I mean, it’s no big deal. There’s no pain, there’s no recovery, you just go home.”
-Nicky, mild® Patient

“By the time I walked out [after the mild® Procedure], I went home, and I could get out. The first thing I wanted to do was start doing stuff. That was a big moment in my life emotionally.”
-Ronnie, mild® Patient
LSS patients who receive a series of epidural injections may become frustrated or start to lose hope if the injections provide little relief or decreasing relief over time. While ESIs can sometimes deliver transient, temporary relief, they do not address the root cause of stenosis. The mild® Procedure decompresses, debulking the hypertrophied ligament, to reduce spinal canal narrowing, giving patients a new option to achieve long-term relief.
Janna L. Friedly et al. studied the long-term effectiveness of ESIs for LSS and the effect of repeat injections. The trial concluded that repeated epidural injections offer no additional benefit if injections in the first 6 weeks did not improve pain.

“If the patient has received ESIs in your practice and the outcomes were not efficacious, there is no reason to offer them another one. If a patient comes to you from another practice, and has recent history of epidural steroid injections, move to mild®. They’re likely seeking an alternate option, and mild® is something that can provide them hope.”

“The first epidural lasted about three months and then the pain was back. I went for the second epidural, and it didn’t last two weeks. My physician said, ‘Well, you can have one more.’ I said, ‘No, I’m finished with them, I want the mild® Procedure.’”
Here are a few tips to help you successfully integrate mild® into your practice.
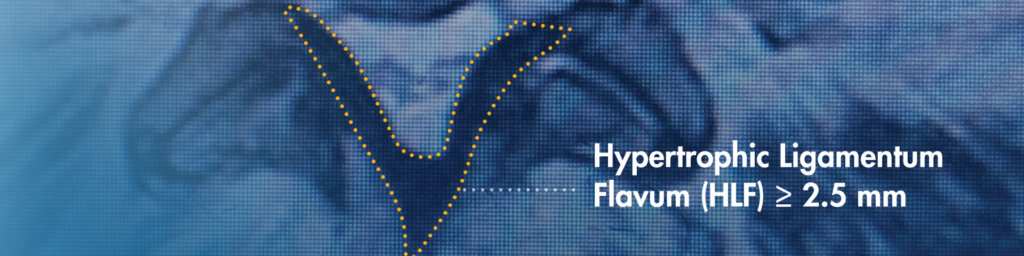
When reviewing a patient’s MRIs/CTs, be sure to identify hypertrophic ligamentum flavum (HLF). HLF ≥ 2.5 mm can be readily identified and is surprisingly common, contributing to up to 85% of spinal canal narrowing.
Introducing patients to mild® early in their treatment journey can help them feel assured that they have effective options if ESIs don’t provide the relief they need. Dr. Jason Pope of the Evolve Restoration Center confirms, “By presenting mild® as part of the treatment plan from the start, patients have more confidence that we are going to be proactive in their care, and make sure they have the opportunity to access advanced LSS treatment options that offer excellent outcomes.”
Many practices that offer mild® ensure that physicians and Advanced Practice Providers (APPs) alike are trained to identify patient candidates who may benefit from the mild® Procedure. Jane Hartigan, an APP in a leading Northern California pain management practice recommends asking proactive questions like, “Does hunching forward significantly relieve the pain you’re feeling?”, or “Do you feel pain in your back and legs when standing or walking?” By understanding your patient’s current experience with LSS, their treatment history, and functional goals, you can readily spot the many patients in your practice who may benefit from the mild® Procedure.
Physicians and practices treating lumbar spinal stenosis (LSS) patients with epidural steroid injections (ESIs) will likely recognize a common “condition” among serial ESI patients that our practice typically refers to as “Epidural Exhaustion.” Epidural Exhaustion is typically seen in patients who have tried multiple injections but have experienced only short-term functional improvements with little to no pain relief. Many times, LSS patients who do not improve after a single ESI or series of ESIs can become frustrated, or begin to lose hope in ever feeling relief from their spinal stenosis symptoms.
While “Epidural Exhaustion” is remarkably common among lumbar spinal stenosis (LSS) patients, it is avoidable. In our practice, we have found that moving to mild® (Minimally Invasive Lumbar Decompression) earlier in the patient journey helps drive positive long-term clinical outcomes and stronger patient satisfaction. Today, we have changed our practice workflows to move more patients to mild® after their first failed ESI. We proactively communicate the benefits of the mild® Procedure to our patients at the time of diagnosis or before they begin their treatment journey in our practice, so that they understand the breadth of their treatment options. With the availability of mild® as an early treatment option for more spinal stenosis patients, we can reduce incidences of ESI exhaustion and put more of our LSS patients on the path to long-term relief.
Although repetitive epidural steroid injections are still the standard of care in many practices and instilled during our training, the data show that serial ESIs offer little benefit to long-term patient outcomes.
As noted by Heidi Younan, Director of Patient Marketing & Practice Integration at Vertos Medical, we already know that epidurals are not capable of “curing” neurogenic claudication, which is present in 94% of patients with lumbar spinal stenosis. While repetitive ESIs deliver some chemical benefit and can offer transient, temporary relief for radicular components, ESIs do not address a major root cause of stenosis. Decompression is required to reduce narrowing/triangulation and relieve pressure in the central canal.
A 2016 study conducted by Janna L. Friedly et al., studied the overall long-term effectiveness of treatment with epidural corticosteroid injections for lumbar central spinal stenosis and the effect of repeat injections on LSS patient outcomes through 12 months. The multicenter, double-blind, randomized, controlled trial with 400 patients concluded that repeated epidural injections offer no additional benefit if injections in the first 6 weeks did not improve pain. Therefore, performing multiple ESIs on patients who did not benefit from the initial injection is essentially delaying treatment that can provide beneficial and long-term outcomes.
Because of its effectiveness and safety profile, I strongly recommend moving to mild® earlier in the patient journey, after the first failed ESI. Recognizing that up to 85% of spinal canal narrowing is the result of hypertrophic ligamentum flavum (HLF), there is tremendous benefit in being able to debulk the HLF and restore space in the spinal canal, thus reducing compression of the nerves. The minimally invasive mild® Procedure enables our practice to offer a treatment option that is a step up from ESIs with the same low complication rate, but with excellent effectiveness and lasting results.
Ms. Younan also highlights that clinical studies of mild® have demonstrated clinically meaningful, statistically significant improvements in mobility, Oswestry Disability Index (ODI), and pain reduction on the numeric pain rating scale (NPRS). More recent 5-year data shows that after undergoing the mild® Procedure, only 12% of patients required surgical decompression at the same level over 5 years. Stated differently, mild® gives the potential to help 88% of patients avoid surgical decompression surgery for at least 5 years, while providing symptomatic relief during that time.


Beyond the individual clinical benefits, I also advise other pain specialists that there are many practice-driven reasons to offer mild® in lieu of serial epidural steroid injections. The MiDAS ENCORE level 1 data demonstrated an exceptionally high 85% patient satisfaction rate among patients who received the mild® Procedure. Patient satisfaction is a key benchmark in our practice. With the mild® Procedure, we have seen tremendous patient satisfaction, and we know that those patients tell their friends, relatives, and neighbors about their positive results, which increases word-of-mouth to our practice.
My goal as a physician is always to assess where the patient is in their treatment journey, the impact of lumbar spinal stenosis symptoms on their lifestyle, their priorities for the procedure (such as a minimally invasive approach or a short recovery time), and their treatment history to determine the best path forward for that individual.
This purpose of this article is certainly not to challenge any and all use of ESIs within the modern pain or spine practice. ESIs can be an effective tool and are a critical part of our practice armamentarium. For some patients, ESIs offer significant benefits; however, evidence and experience demonstrate that ESIs are not a one-size-fits-all solution. Many patients with LSS fail to achieve significant pain reduction, while others who do experience pain reduction or functional improvements find those benefits to have limited durability.
In our practice, we still commonly begin a lumbar spinal stenosis treatment plan with a conservative approach to determine if an ESI will effectively reduce the symptoms of LSS. Once we understand how our patient responds to an ESI, we can determine the best treatment plan for the patient. If they have a good result with an ESI, we suggest that they resume normal activities and self-monitor for changes over time. On the other hand, if they do not see significant benefit from the initial ESI, I will strongly consider them as a candidate for mild®, and barring any contraindications, we typically schedule their mild® Procedure within a few weeks.
A powerful message for patients looking for significant relief is that we will start their LSS treatment with an ESI, but we’ll also be able to know very quickly if the epidural steroid injection is going to be successful for them. When patients understand that they have other minimally invasive, nonsurgical options, they may be less concerned if they experience limited relief from their first ESI.
The key question is, “At what point can we reliably assess whether an individual patient will benefit from serial ESIs?” Here again, we can look to the Friedly data showing that repeated epidural injections offered no additional long-term benefit if injections in the first 6 weeks did not improve pain. Based on this data and the results we’ve seen first-hand in our own practice, we know that we can assess our LSS patient results post-ESI and determine whether we should move them to mild® earlier in their treatment journey.

(Graphic courtesy of Vertos Medical)
One of the most significant ways our practice has shifted after the adoption of mild® is that we now evaluate most patients as potential mild® candidates. If we suspect LSS, we present mild® as a treatment option from the start. In making this shift, we have discovered a few clinical and workflow pearls that optimize success across the practice.
1. Train staff to recognize patient symptoms
Because LSS is especially common among adults over 60 years of age, and our practice treats a high volume of senior patients, we have found that many of our existing and new patients are excellent candidates for mild®.
Our practice trains our APPs and other support staff so they can immediately recognize the signs and symptoms of LSS independently. Of course, we ultimately diagnose LSS and neurogenic claudication with the support of imaging, but I always remind our staff and patients that imaging can’t show pain. As a healthcare team, we need to be proactively asking questions like, “Does hunching forward significantly relieve the pain you’re feeling?” or “Do you feel pain in your back and legs when standing or walking?” If the answer to either question is yes, we know there’s a good chance that patient may be candidate for mild®.
Younan also notes that the Vertos team has developed resources and training to support APPs. All resources are available through your Vertos sales representative.
2. Look for the ligament
When reviewing a patient’s MRIs/CTs, a simple rule we use is “look for the ligament.” Hypertrophic ligamentum flavum is a common problem that contributes up to 85% of spinal canal narrowing. We have trained our APPs to apply this rule while reviewing images to help identify patients who may benefit from the mild® Procedure.

In the image, the thickened ligament can be seen clearly, resulting in compression of the spinal cord nerves.
3. Present mild® in your initial LSS treatment plan
When we work with patients, we make sure they know that our focus is on improving their functionality and reducing pain. We are clear from the start that we’re going to evaluate their symptoms, treatment history, and goals to determine their best treatment pathway based on the latest evidence and techniques.
Patients are generally open to the idea that a therapy that works for one patient might not work for them. So, I typically tell the patient, “We are going to start with a single epidural, and we’ll be able to know quickly if the epidural is going to be effective for you.” I also let them know that if they do not experience a significant improvement after the epidural, or if those results wear off after a short period, we can still help them with proven, minimally invasive options. Then, I tell them about the mild® Procedure, and how it offers a safety profile similar to an epidural, but with lasting results.
By presenting mild® as part of the treatment plan from the start, patients have more confidence that we are going to be proactive in their care and make sure they have the opportunity to access advanced LSS treatment options that offer excellent outcomes.
Overall, the addition of mild® has been a real game-changer in our practice. We see mild® as a safe and effective alternative to serial ESIs that drive long-term patient improvements and high patient satisfaction—a treatment we make available to as many patients as possible, as early as possible. I regularly tell other interventional pain specialists to incorporate mild® in their practice, and once they see the impact it has on their patients, they understand why we are so passionate about moving to mild® after failure of the first ESI.
About Dr. Jason Pope
 Jason Pope, MD, is an Interventional Pain Physician trained in Pain Medicine at the Cleveland Clinic with board certifications in Anesthesia and Pain Medicine. He completed an Anesthesiology Residency at Vanderbilt University Medical Center and received his medical degree from the Indiana University School of Medicine. Dr. Pope is the Founder and CEO of Evolve Restorative Center in Northern California and serves as an expert reviewer for the Medical Board of California. Dr. Pope also serves as President Emeritus and Chairman for the Pacific Spine & Pain Society (PSPS), President Elect for the American Society of Pain & Neuroscience (ASPN), Director at Large and Chairman for the International Neuromodulation Society (INS), and Chairman for the North American Neuromodulation Society (NANS).
Jason Pope, MD, is an Interventional Pain Physician trained in Pain Medicine at the Cleveland Clinic with board certifications in Anesthesia and Pain Medicine. He completed an Anesthesiology Residency at Vanderbilt University Medical Center and received his medical degree from the Indiana University School of Medicine. Dr. Pope is the Founder and CEO of Evolve Restorative Center in Northern California and serves as an expert reviewer for the Medical Board of California. Dr. Pope also serves as President Emeritus and Chairman for the Pacific Spine & Pain Society (PSPS), President Elect for the American Society of Pain & Neuroscience (ASPN), Director at Large and Chairman for the International Neuromodulation Society (INS), and Chairman for the North American Neuromodulation Society (NANS).
About Heidi Younan
 Heidi Younan is the Director of Patient Marketing & Practice Integration at Vertos Medical. A critical member of the Vertos team for over a decade, Ms. Younan works with clinicians, APPs, and patients to support the successful integration of the mild® Procedure in pain practices and help more patients experience the life-changing benefits of mild®. Ms. Younan also contributed portions of the clinical content within this article.
Heidi Younan is the Director of Patient Marketing & Practice Integration at Vertos Medical. A critical member of the Vertos team for over a decade, Ms. Younan works with clinicians, APPs, and patients to support the successful integration of the mild® Procedure in pain practices and help more patients experience the life-changing benefits of mild®. Ms. Younan also contributed portions of the clinical content within this article.
The objective of the study “Use of Epidurogram is Not Necessary for Safe, Minimally Invasive Direct Lumbar Decompression” was to investigate the safety of using osteal landmarks vs an epidurogram to establish a visual safety barrier prior to decompression with the mild® Procedure. A retrospective data analysis was performed on 147 patients that compared those receiving an epidurogram with performance of the mild® Procedure versus those that did not. View the abstract poster below to learn more about the outcomes.

Watch Dr. Jason Pope present his abstract from the American Society of Pain and Neuroscience’s (ASPN) Third Annual Conference where he shares why the use of an epidurogram is not necessary for safe decompression with the mild® Procedure.
Eager to further understand the mild® Procedure and how it can put your lumbar spinal stenosis patients on the path to long-term relief? Contact Vertos Medical and discover why leading interventionalists offer mild® in their practice.
This study was recently published in the Journal of Pain Research, click here to view.
Jason Pope, MD (00:00)
Well, welcome everyone. My name is Dr. Jason Pope. I have a practice in Northern California serving Napa, Sonoma, Mendocino counties and I’m here today to talk a little bit about an innovative strategy that we had in performing the mild® Procedure. And the question that we were trying to answer with this was essentially, “Is the epidurogram necessary when executing the mild® Procedure on patients, whether you’re going to perform a single level or a multilevel strategy?” And so we retrospectively looked at 147 patients. So we can see here the female to male breakdown along with a median age of around 77. And we looked at patients that either got the epidurogram with performance of the mild® [Procedure] versus those that did not. And we did so equitably across the patient population that we were serving. And as we can see with a stenotic level, clearly the most common areas that were performed were at L3-4 and L4-5 and that’s no surprise. And then we looked at patients whether they were done as a single level or a multilevel. And we can see here that the breakdown of these patients where 54 patients received contrast, 42 non-contrast, in a single level. And the most common level that was performed was at L4-5. But what was also very interesting was in this retrospective review, again, of about 147 patients, about 80% of them had the procedure done bilaterally if it was at a single level. And interestingly enough, when we did a multilevel mild®, and in this cohort, it represented at least two levels, the most common level that was the index level of where it was treated was at L3-4 and bilaterally, this was performed nearly 50% of the time. So when people do multiple levels of [the] mild® Procedure, the unilateral reality based on symptoms changes a little bit as compared to if you were doing a single level.
(02:27) And again, this was done over a total cohort of 147 patients. And the way that this came about and again, we highlighted the fact that when we looked at the use of epidurogram in managing patients with spinal stenosis symptomatically, with the mild® Procedure, it wasn’t clear that it really offered an increase in safety. And it wasn’t clear that it highlighted when the decompression was completed because epidural flow after decompression doesn’t necessarily increase after the ligament is trimmed or resected. So we looked at trying to simplify the procedure, and this was done just by using the osteal landmarks. And we can see the facet line and the laminar line in the middle section of the abstract presentation here. And I can say with confidence, using osteal landmarks alone as compared to the epidurogram, and we use the midline incision, the refined Streamlined Technique that you all have come to know and love, that in performing this, there were zero complications, as defined as problems with infection, nerve injury, allergy to contrast, which is something that can happen, but clearly in this cohort, it did not.
And so there were zero complications performing either one level or multilevel [mild®], either unilaterally or bilaterally with contrast or without contrast. So this underscores the importance and the strategy that if one chooses or elects to not use the epidurogram in performance of the mild® [Procedure] you can do so safely, based on this patient cohort. So again, appreciate your time. I want to also thank my co-authors associated with this project, Dr. Timothy Deer and Dr. Steven Falowski. And I want to also thank the Vertos team for helping with the assembly of the abstract. So with that, thank you.
Benyamin RM, Staats PS, MiDAS ENCORE Investigators. mild® is an effective treatment for lumbar spinal stenosis with neurogenic claudication: MiDAS ENCORE Randomized Controlled Trial. Pain Physician. 2016;19(4):229-242.
Mekhail N, Costandi S, Abraham B, Samuel SW. Functional and patient-reported outcomes in symptomatic lumbar spinal stenosis following percutaneous decompression. Pain Pract. 2012;12(6):417-425. doi:10.1111/j.1533-2500.2012.00565.x.
2012 data from Health Market Sciences report for Vertos Medical 2013.
Data on file with Vertos Medical.
Staats PS, Chafin TB, Golvac S, et al. Long-term safety and efficacy of minimally invasive lumbar decompression procedure for the treatment of lumbar spinal stenosis with neurogenic claudication: 2-year results of MiDAS ENCORE. Reg Anesth Pain Med. 2018;43:789-794. doi:10.1097/AAP.0000000000000868.
Based on mild® Procedure data collected in all clinical studies. Major complications are defined as dural tear and blood loss requiring transfusion.
MiDAS ENCORE responder data. On file with Vertos Medical.
Jain S, Deer TR, Sayed D, et al. Minimally invasive lumbar decompression: a review of indications, techniques, efficacy and safety. Pain Manag. 2020;10(5). https://doi.org/10.2217/pmt-2020-0037. Accessed June 1, 2020.
Deer TR, Grider JS, Pope JE, et al. The MIST Guidelines: the Lumbar Spinal Stenosis Consensus Group guidelines for minimally invasive spine treatment. Pain Pract. 2019;19(3)250-274. doi:10.1111/papr.12744.
Hansson T, Suzuki N, Hebelka H, Gaulitz A. The narrowing of the lumbar spinal canal during loaded MRI: the effects of the disc and ligamentum flavum. Eur Spine J. 2009;18(5):679-686. doi:10.1007/s00586-009-0919-7.
Treatment options shown are commonly offered once conservative therapies (e.g., physical therapy, pain medications, chiropractic) are not providing adequate relief. This is not intended to be a complete list of all treatments available. Doctors typically recommend treatments based on their safety profile, typically prioritizing low risk/less aggressive procedures before higher risk/more aggressive procedures, but will determine which treatments are appropriate for their patients.
The mild® Procedure is a minimally invasive treatment for lumbar spinal stenosis. As with most surgical procedures, serious adverse events, some of which can be fatal, can occur, including heart attack, cardiac arrest (heart stops beating), stroke, and embolism (blood or fat that migrates to the lungs or heart). Other risks include infection and bleeding, spinal cord and nerve injury that can, in rare instances, cause paralysis. This procedure is not for everyone. Physicians should discuss potential risks with patients. For complete information regarding indications for use, warnings, precautions, and methods of use, please reference the devices’ Instructions for Use.
Patient stories on this website reflect the results experienced by individuals who have undergone the mild® Procedure. Patients are not compensated for their testimonial. The mild® Procedure is intended to treat lumbar spinal stenosis (LSS) caused by ligamentum flavum hypertrophy. Although patients may experience relief from the procedure, individual results may vary. Individuals may have symptoms persist or evolve or other conditions that require ongoing medication or additional treatments. Please consult with your doctor to determine if this procedure is right for you.
Reimbursement, especially coding, is dynamic and changes every year. Laws and regulations involving reimbursement are also complex and change frequently. Providers are responsible for determining medical necessity and reporting the codes that accurately describe the work that is done and the products and procedures that are furnished to patients. For this reason, Vertos Medical strongly recommends that you consult with your payers, your specialty society, or the AMA CPT regarding coding, coverage and payment.
Vertos Medical cannot guarantee coding, coverage, or payment for products or procedures. View our Billing Guide.
Vertos is an equal employment opportunity workplace committed to pursuing and hiring a diverse workforce. We strive to grow our team with highly skilled people who share our culture and values. All qualified applicants will receive consideration for employment without regard to sex, age, color, race, religion, marital status, national origin, ancestry, sexual orientation, gender identity, physical & mental disability, medical condition, genetic information, veteran status, or any other basis protected by federal, state or local law.
Hall S, Bartleson JD, Onofrio BM, Baker HL Jr, Okazaki H, O’Duffy JD. Lumbar spinal stenosis. Clinical features, diagnostic procedures, and results of surgical treatment in 68 patients. Ann Intern Med. 1985;103(2):271-275. doi:10.7326/0003-4819-103-2-271.
Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence & association with symptoms: The Framingham Study. Spine J. 2009;9(7):545-550. doi:10.1016/j.spinee.2009.03.005.
Fukusaki M, Kobayashi I, Hara T, Sumikawa K. Symptoms of spinal stenosis do not improve after epidural steroid injection. Clin J Pain. 1998;14(2):148-151. doi:10.1097/00002508-199806000-00010.
Mekhail N, Costandi S, Nageeb G, Ekladios C, Saied O. The durability of minimally invasive lumbar decompression procedure in patients with symptomatic lumbar spinal stenosis: Long-term follow-up [published online ahead of print, 2021 May 4]. Pain Pract. 2021;10.1111/papr.13020. doi:10.1111/papr.13020
Friedly JL, Comstock BA, Turner JA, et al. Long-Term Effects of Repeated Injections of Local Anesthetic With or Without Corticosteroid for Lumbar Spinal Stenosis: A Randomized Trial. Arch Phys Med Rehabil. 2017;98(8):1499-1507.e2. doi:10.1016/j.apmr.2017.02.029
Pope J, Deer TR, Falowski SM. A retrospective, single-center, quantitative analysis of adverse events in patients undergoing spinal stenosis with neurogenic claudication using a novel percutaneous direct lumbar decompression strategy. J Pain Res. 2021;14:1909-1913. doi: 10.2147/JPR.S304997
Pryzbylkowski P, Bux A, Chandwani K, et al. Minimally invasive direct decompression for lumbar spinal stenosis: impact of multiple prior epidural steroid injections [published online ahead of print, 2021 Aug 4]. Pain Manag. 2021;10.2217/pmt-2021-0056. doi:10.2217/pmt-2021-0056
Abstract presented at: American Society of Pain and Neuroscience Annual Conference; July 22-25, 2021; Miami Beach, FL.
Mobility Matters: Low Back Pain in America, Harris Poll Survey, 2022. View data and full summary here.
Deer TR, Grider JS, Pope JE, et al. Best Practices for Minimally Invasive Lumbar Spinal Stenosis Treatment 2.0 (MIST): Consensus Guidance from the American Society of Pain and Neuroscience (ASPN). J Pain Res. 2022;15:1325-1354. Published 2022 May 5. doi:10.2147/JPR.S355285.